Balancing Chemical Equations
Related Pages
Writing Chemical Equations
Ionic Equations
More lessons on Chemistry
In these lessons, we will look at some examples of balancing chemical equations containing polyatomic ions.
Balancing chemical equations is a fundamental skill in chemistry that ensures the Law of Conservation of Mass is upheld. This law states that matter cannot be created or destroyed in a chemical reaction. Therefore, the number of atoms of each element must be the same on both sides of a chemical equation.
The sum of atoms of each element before reaction = the sum of atoms of each element after reaction
The following figure gives some hints on how to balance chemical equations. Scroll down the page for more examples and solutions.
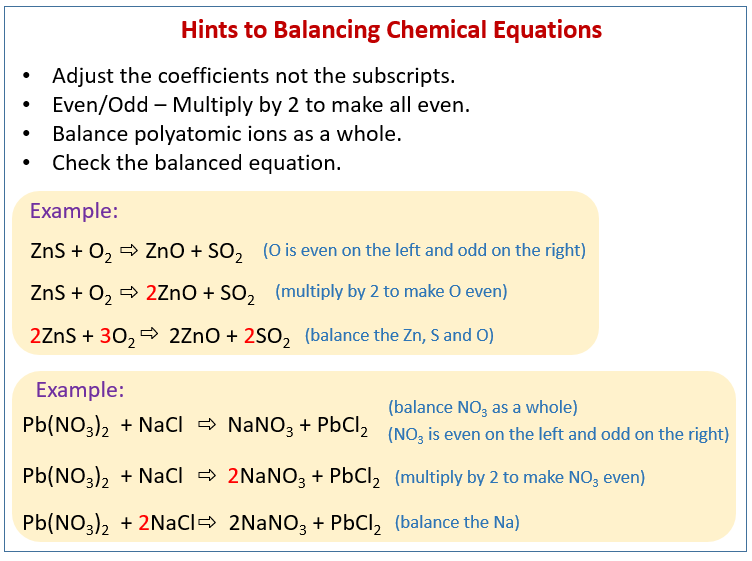
How to balance a chemical equation
- Never change subscripts (e.g., H2O → H3O is wrong). The subscripts tell you how many atoms of that element are in one molecule of the compound.
- Only adjust coefficients (numbers in front of molecules). The coefficients tell you how many molecules of that substance are involved in the reaction. A coefficient applies to every atom in the molecule it precedes.
- Balance one element one by one.
a) Start with elements that appear in only one reactant and one product.
b) If a polyatomic ion appears unchanged on both sides of the equation, treat it as a single unit rather than balancing its individual atoms.
c) Save hydrogen (H) and oxygen (O) for last. They often appear in many compounds, and balancing other elements first will often automatically balance some of the H and O atoms. - Adjust coefficients until all atoms are balanced.
This is an iterative process. You might need to go back and forth, adjusting coefficients. - Check your work.
Once you think the equation is balanced, do a final count of each type of atom on both the reactant and product sides. They must be equal. - Ensure coefficients are in the lowest possible whole-number ratio.
Sometimes you might end up with coefficients like 2, 4, 2. These should be reduced to 1, 2, 1 by dividing all coefficients by the greatest common divisor.
General Rules for balancing chemical equations – Polyatomic Ions
Balancing chemical equations may require some trial and error. There are some general rules that could be helpful, but they may not work all the time.
Rule 1
Balancing chemical equations using the one’s and two’s technique
Rule 2
Balancing chemical equations using the two’s and three’s technique
Rule 3
Balancing chemical equations using the CHO technique
Rule 4
Balancing chemical equations using the even technique
Rule 5
Balancing chemical equations containing polyatomic ions
Case 1: If the polyatomic ion remains the same before and after the reaction, then treat it as “a single element” for ease of calculation. Try to start with the most complicated-looking group.
Example:
Balance the equation
Ca + HNO3 → H2 + Ca(NO3)2
Solution:
- The nitrate ion NO3 is unchanged before and after the reaction.
- Start with Ca(NO3)2 since it looks most complicated.
- There is one NO3 on the left and two on the left.
- Using the one’s and two’s technique, the equation is balanced by placing the coefficient of 2 for HNO3 Ca + 2HNO3 → H2 + Ca(NO3)2
- We test the other atoms and we find that we already have a balanced equation.
- Check to make sure that all coefficients are in the lowest-possible ratio
How to balance chemical equations containing polyatomic ions?
Example:
Balance the following equation:
Ca(NO3)2 + Na2S → CaS + NaNO3
Example:
Balance the following chemical equation:
Fe(NO3)3 + (NH4)2CO3 → Fe2(CO3)3 + NH4NO3
Example:
Balance the following chemical equation:
AgNO3 + K2CrO4 → Ag2CrO4 + KNO3
How to balance chemical equations containing polyatomic ions?
Example:
Balance the following chemical equation:
Pb(NO3)2 + NA2CO3 → PbCO3 + NaNO3
Case 2: If the polyatomic ion is changed after the reaction then it would be necessary to consider each atom individually.
Example:
Balance the chemical equation
Ba(OH)2 + H3PO4 → BaHPO4 + H2O
Solution:
- Use the CHO technique. Since we don’t have carbon, we could try to balance the hydrogen first.
- We have five hydrogen atoms on the left and three hydrogen atoms on the right.
- To balance the hydrogen, we can place the coefficient of 2 at H2O Ba(OH)2 + H3PO4 → BaHPO4 + 2H2O
- We test the other atoms and we find that we already have a balanced equation.
- Check to make sure that all coefficients are in the lowest-possible ratio.
How to balance chemical equations with polyatomic ions by rewriting H2O as H(OH)?
Examples:
Balance the following chemical equations:
Al4C3 + H2O → Al(OH)3 + CH4
C3H8 + O2 → CO2 + H2O
C6H6 + O2 → CO2 + H2O
Three helpful tips and tricks that make Balancing Chemical Equations easier
Tip #1: Put a star(*) next to any element appearing more than once on either the product or the reactant side; balance those elements last.
Tip #2: Recognize polyatomic groups that appear on both sides of the equation, and tea them as single items (e.g. don’t break SO4 into one sulfur and four oxygens)
Tip #3: If you have H and OH on one side and H2O on the other, it is helpful to rewrite H2) as H(OH).
Examples:
Balance the following chemical equations:
- C3H8 + O2 → CO2 + H2O
- Al2(SO4)3 + Ca(OH)2 → Al(OH)3 + CaSO4
- H3PO4 + NaOH → Na3PO4 + H2O
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.