Writing A Balanced Chemical Equation
Related Pages
Writing Ionic Equations
Molar Volume, Avogadro’s Law
Chemistry Lessons
In these lessons, we will learn how to write a balanced chemical equation given the word equation. We have more lessons on the rules for balancing chemical equations.
Chemical Equation
A chemical equation shows the overall change of reactants to products in a chemical reaction. The reactants are written on the left side of the arrow and the products are written on the right side of the arrow. The arrow indicates the direction of the reaction.

Sometimes, state symbols are required to indicate the physical states of the substances in a chemical reaction.
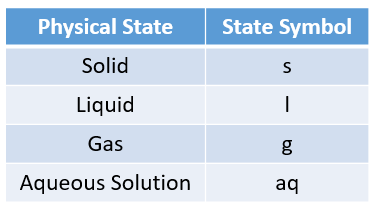
The following table gives the physical states and the state symbols used in chemical equations: solid, liquid, gas, aqueous.
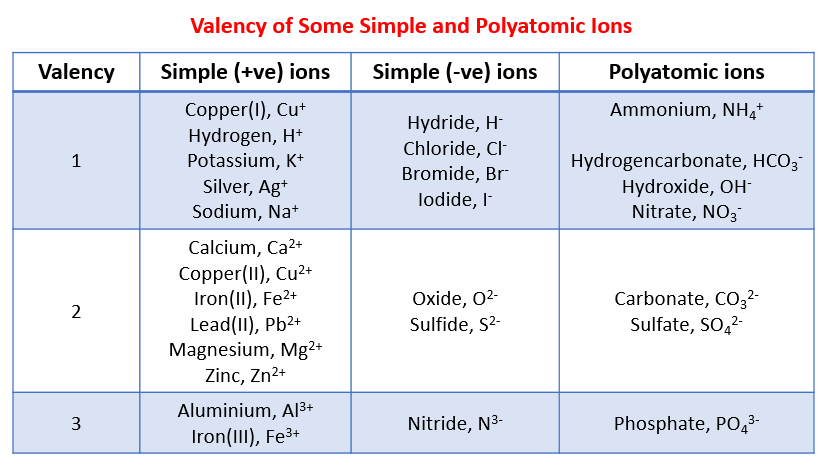
The following table gives the valency of some common ions. This table can be used to help you work out the chemical formula of the reactants and products.
Here are some simple covalent formulas that you will find useful to remember:
- Water H2O,
- Carbon Dioxide CO2,
- Ammonia NH3,
- Hydrogen H2,
- Oxygen O2,
- Nitrogen N2,
- Sulfur Dioxide or Sulphur Dioxide SO2,
- Methane CH4
Here are some simple ionic formulas that you will find useful to remember:
- Sodium Chloride NaCl,
- Calcium Chloride CaCl2,
- Magnesium Oxide MgO,
- Hydrochloric Acid HCl,
- Sulfuric Acid or Sulphuric Acid H2SO4,
- Nitric Acid HNO3,
- Sodium Hydroxide NaOH,
- Potassium Hydroxide KOH,
- Calcium Hydroxide Ca(OH)2,
- Calcium Carbonate CaCO3,
- Aluminum Oxide Al2O3,
- Iron Oxide Fe2O3
The following diagram shows how to write a chemical equation. Scroll down the page for more examples and solutions.
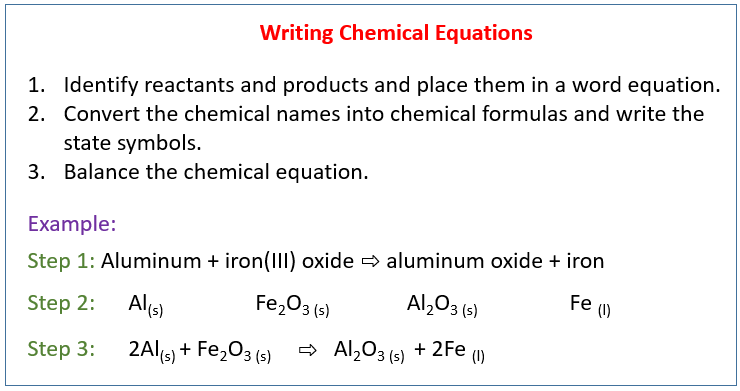
Conversion Of Word Equation To Chemical Equation
Example:
In a precipitation reaction, sodium hydroxide solution is mixed with iron(II) chloride solution.
Sodium Chloride solution and insoluble iron(II) hydroxide are produced. Write a balanced chemical
equation including the state symbols.
Solution:
Step 1: Identify reactants and products and place them in a
word equation.
sodium hydroxide + iron(II) chloride → sodium chloride + iron(II) hydroxide
Step 2: Convert the chemical names into chemical formulas. Place them based on the chemical equation and write the state symbols.
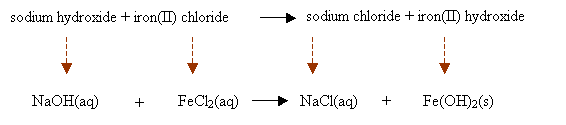
Step 3: Balance the chemical equation.
2NaOH(aq) + FeCl2(aq) → 2NaCl(aq) + Fe(OH)2(s)
Example:
Write a balanced chemical equation for
Sodium(s) + hydrochloric acid(aq) → sodium chloride(aq) + hydrogen(g)
Solution:
Step 1: Convert the chemical names into chemical formulas.
Place them based on the chemical equation and write the state symbols.

Step 2: Balance the chemical equation.
2Na(s) + 2HCl(aq) → 2NaCl(aq) + H2(g)
How To Write A Balanced Chemical Equation From A Word Equation?
When compounds react, they are chemically changed into new compounds. Every chemical change can be communicated symbolically using a chemical equation. Chemical equations combine formulas with other symbols to show what changes takes place.
Examples:
Aluminum + Iron(III) oxide → Aluminum oxide + Iron
Oxygen + Hydrogen → Water
Methane + Oxygen → Carbon Dioxide + Water
Butane + Oxygen → Carbon Dioxide + Water
How To Interpret Chemical Symbols And Write Simple Balanced Equations?
Each element is represented by a different symbol.
All these symbols are in the periodic table.
We can use these symbols to show molecules of compounds, and they can show us the ratio of the
different elements which combine to form compounds.
Practice Writing Chemical Equations From Word Problems And Balancing Equations
Examples:
- Ammonium nitrate decomposes explosively to form nitrogen, oxygen, and water vapor.
- Dinitrogen tetrahydride reacts with oxygen to produce nitrogen and water.
- Lead(II) nitrate reacts with sodium iodide to create lead (II) iodide and sodium nitrate.
- Phosphorous reacts with oxygen gas to produce diphosphorous pentoxide.
- When calcium comes in contact with water, calcium hydroxide and hydrogen gas is produced.
- When hexane (C6H24) reacts with oxygen a combustion reaction occurs. This reaction produces carbon dioxide and water.
- Sodium hydroxide reacts with iron (III) nitrate to create a precipitate of iron (III) hydroxide in a solution of sodium nitrate.
- Mercury (II) oxide decomposes to produce mercury and oxygen.
- Zinc hydroxide reacts with phosphoric acid (H3PO4) to produce zinc phosphate and water.
- Sulfur dioxide and oxygen combine to produce sulfur trioxide.
Tips for Balancing Chemical Equations:
- Polyatomic Ions: If a polyatomic ion (like SO4, NO3, PO4) appears unchanged on both sides of the equation, treat it as a single unit. This can greatly simplify the balancing process.
- Odd and Even Numbers: If you have an element that’s odd on one side and even on the other, try putting a coefficient of ‘2’ in front of the compound with the odd number of atoms. This often makes it even and easier to balance.
- Trial and Error: Balancing sometimes involves a bit of trial and error, especially for more complex equations. Don’t be afraid to erase and try different coefficients.
- Fractions (Temporary): While coefficients must be whole numbers in the final equation, some people find it helpful to temporarily use fractions (e.g., 1/2 O2). If you do, multiply the entire equation by the denominator to get whole numbers at the end.
- Practice: The more you practice, the easier and faster balancing equations will become.
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.