Mole Calculation
In these lessons, we will learn
- how to calculate the mass of a substance when we are given the number of moles (mole to mass conversion).
- how to calculate the number of moles of a substance when we are given the mass (mass to mole conversion).
Related Pages
Molar Volume
Stoichiometry Lessons
Molecular Mass
Writing A Balanced Chemical Equation
Chemistry Lessons
The following diagram shows the conversion between Mole and Mass. Scroll down the page for more examples and solutions.
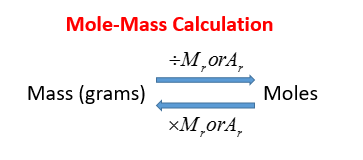
Mole-Mass Equation
mass = number of moles × molar mass
where mass is in grams and the molar mass is in grams per mole.
Moles to Mass Calculation
We can use the above equation to find the mass of a substance when we are given the number of moles of the substance.
Example:
Calculate the mass of
(a) 2 moles and
(b) 0.25 moles of iron. (Relative atomic mass: Fe = 56)
Solution:
a) mass of 2 moles of iron
= number of moles × molar mass
= 2 × 56
= 112 g
b) mass of 0.25 mole of iron
= number of moles × molar mass
= 0.25 × 56
= 14 g
Example:
Calculate the mass of
(a) 3 moles and
(b) 0.2 moles of carbon dioxide gas, CO2. (Relative atomic mass: C = 12; O = 16)
Solution:
a) mass of 1 mole of CO2
= (1 × 12) + (2 × 16)
= 44 g
mass of 3 moles of CO2
= 3 × 44
= 132g
b) mass of 0.2 mole of CO2
= 0.2 × 44
= 8.8 g
Examples of moles to mass calculation
Example:
If an experiment calls for 0.200 mol acetic acid (HC2H3O2),
how many grams of glacial acetic acid do we need?
Formula: m = nM
Example:
If an experiment calls for 0.500 mol CaCO3, how many grams of pure calcium carbonate do we need?
Mass to Moles Calculation
If we are given the mass of a substance and we are asked to find the number of moles of the substance,
we can rewrite the above equation as
![]()
Example:
Calculate the number of moles of aluminum present in
(a) 108 g and
(b) 13.5 g of the element. (Relative atomic mass: Al = 27)
Solution:
a) 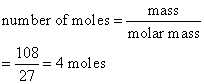
b) 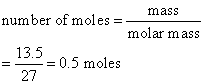
Example:
Calculate the number of moles of magnesium oxide, MgO in
(a) 80 g and
(b) 10 g of the compound. (Relative atomic mass: O = 16, Mg = 24)
Solution:
a) Mass of 1 mole of MgO
= (1 × 24) + (1 × 16)
= 40 g
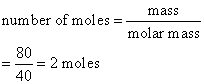
b) 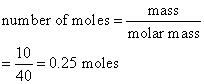
Examples of mass to mole calculation
How many moles of acetic acid (HC2H3O2) are present in a 5.00 g sample of pure acetic acid?
How to use formula mass to convert grams to moles and moles to grams?
Examples:
- How many moles of NAOH are represented by 80.0 grams of NAOH?
- How many grams will 3.5 moles of NAOH weigh?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.