

Scientific Notation in Chemistry
A series of free High School Chemistry
In this lesson, we will learn
There are certain unit systems which the scientific community has universally agreed upon.
To measure distance in scientific units we use meters (m),
to measure mass we use grams (g),
to measure volume we use liters (L) and
to measure temperature we use either degrees centigrade (C) or degrees Kelvin (K).
Units, SI, and Metric Prefix Conversions
The following figure shows how to convert to scientific notation. Scroll down the page for more examples and solutions.
Scientific notation is used to make extremely large or small numbers more manageable. Numbers written in scientific notation are the products of a digit term and an exponential term and are written in the general form a x 10n. For example, 0.0000234 is written 2.34 × 10n and 456,000 is written as 4.56 × 105.
Learn to convert numbers into and out of scientific notation. Scientific notation is a way to express very big and very small numbers with exponents as a power of ten. It is also sometimes called exponential notation. In this video, we will have an explanation and tutorial, and do practice and example problems.
Scientific Notation Practice Problems
We'll do lots of practice problems so that scientific notation becomes really easy. In scientific notation, you express a number with exponents on ten. As you move the decimal point, the exponent on 10 changes. Scientific notation is sometimes called exponential notation. Significant Figures
When working with scientific data, we only want to show as many figures as carry accurate meaning, called significant figures. When adding or subtracting two numbers, we round to the same number of decimal places as the term with the fewest decimal places. When multiplying or dividing numbers we round to the same number of figures as the term with the lowest number of significant figures. In scientific notation, the digit term, not the exponential term counts as significant.
Significant Figures and Zero
When you're doing significant digits, how do you deal with zeros? Significant Zero Practice Problems
Lots of practice problems to work on significant figures with zeros. How to round with significant figures when you're doing scientific notation problems?



In this lesson, we will learn
- Scientific Units
- Scientific Notation
- Significant Figures
There are certain unit systems which the scientific community has universally agreed upon.
To measure distance in scientific units we use meters (m),
to measure mass we use grams (g),
to measure volume we use liters (L) and
to measure temperature we use either degrees centigrade (C) or degrees Kelvin (K).
Units, SI, and Metric Prefix Conversions
The following figure shows how to convert to scientific notation. Scroll down the page for more examples and solutions.
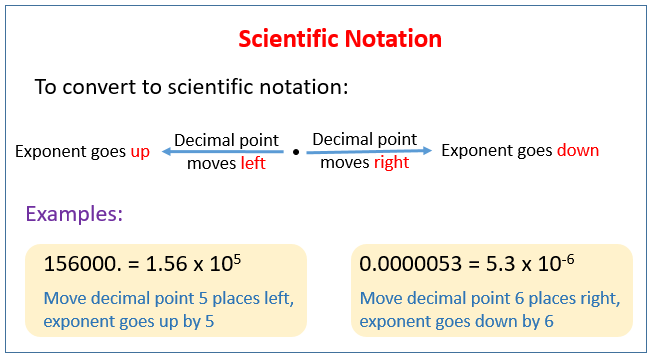
Scientific notation is used to make extremely large or small numbers more manageable. Numbers written in scientific notation are the products of a digit term and an exponential term and are written in the general form a x 10n. For example, 0.0000234 is written 2.34 × 10n and 456,000 is written as 4.56 × 105.
Learn to convert numbers into and out of scientific notation. Scientific notation is a way to express very big and very small numbers with exponents as a power of ten. It is also sometimes called exponential notation. In this video, we will have an explanation and tutorial, and do practice and example problems.
We'll do lots of practice problems so that scientific notation becomes really easy. In scientific notation, you express a number with exponents on ten. As you move the decimal point, the exponent on 10 changes. Scientific notation is sometimes called exponential notation. Significant Figures
When working with scientific data, we only want to show as many figures as carry accurate meaning, called significant figures. When adding or subtracting two numbers, we round to the same number of decimal places as the term with the fewest decimal places. When multiplying or dividing numbers we round to the same number of figures as the term with the lowest number of significant figures. In scientific notation, the digit term, not the exponential term counts as significant.
Significant Figures and Zero
When you're doing significant digits, how do you deal with zeros? Significant Zero Practice Problems
Lots of practice problems to work on significant figures with zeros. How to round with significant figures when you're doing scientific notation problems?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.