

Organic Chemistry
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
The unique properties of carbon, Alkanes, Alkenes, Hydrocarbon structure and isomerism, Chemical reactions of the alkanes, Chemical reactions of the alkenes, Alcohols, The reactions of ethanol, Organic acids and esters.
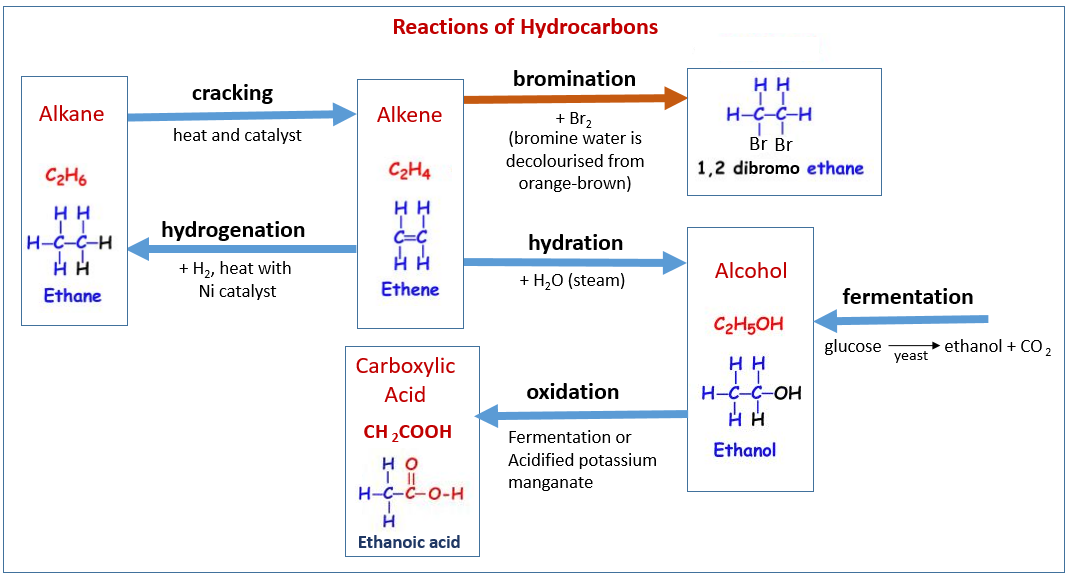
The following diagram shows some of the reactions of hydrocarbons: alkanes, alkenes, alcohol, carboxylic acids and how they are related. Scroll down the page for examples and explanations.
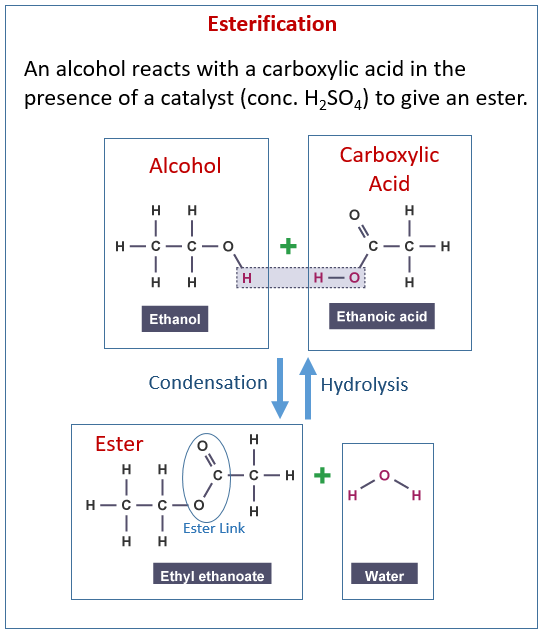
The following diagram shows the reactions of alcohol and carboxylic acids to make esters. Scroll down the page for examples and explanations.
An introduction to the properties of carbon. Hydrocarbons
Alkanes: methane, ethane and propane and the general formula for alkanes. Properties of hydrocarbons
How the length of the carbon chain affects the boiling point, the volatility and the flammability. Unsaturated hydrocarbons
Alkenes: the general formula and how we test for unsaturated hydrocarbons using bromine water. Alkanes isomerism
Isomers in Alkenes
Complete combustion of hydrocarbons
Partial combustion of Hydrocarbons
What happens when hydrocarbons undergo combustion in the presence of limited oxygen.
This is called partial (or incomplete) combustion and it generates the highly toxic gas carbon monoxide, plus water.
Pollution from combustion
How combustion of hydrocarbons causes pollution?
The effects of these pollutants.
This includes global dimming, which examiners seem to like but students rarely seem to understand. Reactions of Alkanes
1. Combustion
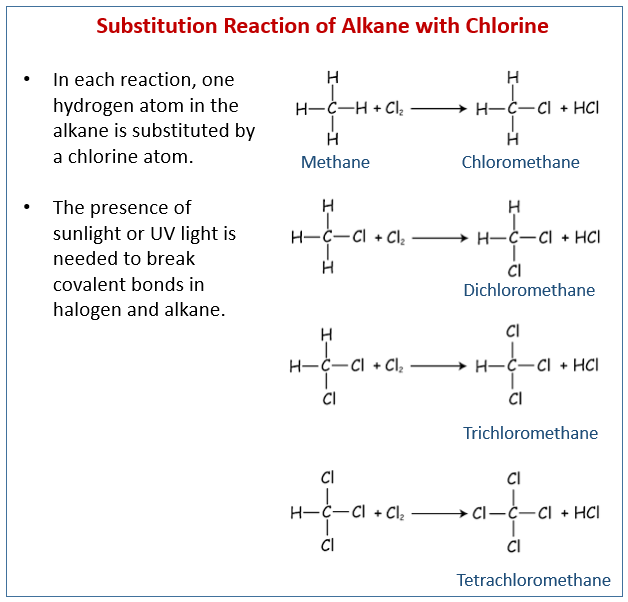
2. Substitution reaction by a Halogen in the presence of light
3. Cracking an Alkane Addition reactions of Alkenes
1. Addition of Hydrogen - Hydrogenation
2. Addition of water - Hydration
3. Addition of Bromine- Bromination Alcohols
The first three alcohols methanol, ethanol and propanol.
The structures of these molecules and their reactions. Making ethanol
How ethanol is made by two processes: hydration of ethene and fermentation of sugar.
Evaluate these two processes to compare the positives and negatives of each. Reactions of ethanol
1. Ethanol as a fuel
2. Oxidation
3. Dehydration
4. Esterification Carboxylic acids
How to make ethanoic acid?
Esters
What are esters?
Esterification and the naming of esters. Hydrolysis and How It Is Used to Make Soaps



More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
The unique properties of carbon, Alkanes, Alkenes, Hydrocarbon structure and isomerism, Chemical reactions of the alkanes, Chemical reactions of the alkenes, Alcohols, The reactions of ethanol, Organic acids and esters.
The following diagram shows some of the reactions of hydrocarbons: alkanes, alkenes, alcohol, carboxylic acids and how they are related. Scroll down the page for examples and explanations.
An introduction to the properties of carbon. Hydrocarbons
Alkanes: methane, ethane and propane and the general formula for alkanes. Properties of hydrocarbons
How the length of the carbon chain affects the boiling point, the volatility and the flammability. Unsaturated hydrocarbons
Alkenes: the general formula and how we test for unsaturated hydrocarbons using bromine water. Alkanes isomerism
Isomers in Alkenes
Complete combustion of hydrocarbons
Partial combustion of Hydrocarbons
What happens when hydrocarbons undergo combustion in the presence of limited oxygen.
This is called partial (or incomplete) combustion and it generates the highly toxic gas carbon monoxide, plus water.
Pollution from combustion
How combustion of hydrocarbons causes pollution?
The effects of these pollutants.
This includes global dimming, which examiners seem to like but students rarely seem to understand. Reactions of Alkanes
1. Combustion
2. Substitution reaction by a Halogen in the presence of light
3. Cracking an Alkane Addition reactions of Alkenes
1. Addition of Hydrogen - Hydrogenation
2. Addition of water - Hydration
3. Addition of Bromine- Bromination Alcohols
The first three alcohols methanol, ethanol and propanol.
The structures of these molecules and their reactions. Making ethanol
How ethanol is made by two processes: hydration of ethene and fermentation of sugar.
Evaluate these two processes to compare the positives and negatives of each. Reactions of ethanol
1. Ethanol as a fuel
2. Oxidation
3. Dehydration
4. Esterification Carboxylic acids
How to make ethanoic acid?
Esters
What are esters?
Esterification and the naming of esters. Hydrolysis and How It Is Used to Make Soaps
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.