Relative Molecular Mass & Relative Formula Mass
Related Pages
Mole Calculation
Stoichiometry Lessons
Chemistry Lessons
In these lessons, we will learn
- how to calculate the relative formula mass or relative molecular mass,
- how to use the relative molecular mass to calculate the percent mass of an element in a compound,
- how to use the relative molecular mass to calculate the percent mass of water in a compound.
The relative molecular mass (Mr) is the sum of the relative atomic masses (Ar) of all the atoms in a molecule.
The following diagram shows how to calculate the relative molecular mass or relative formula mass. Scroll down the page for more examples and solutions.
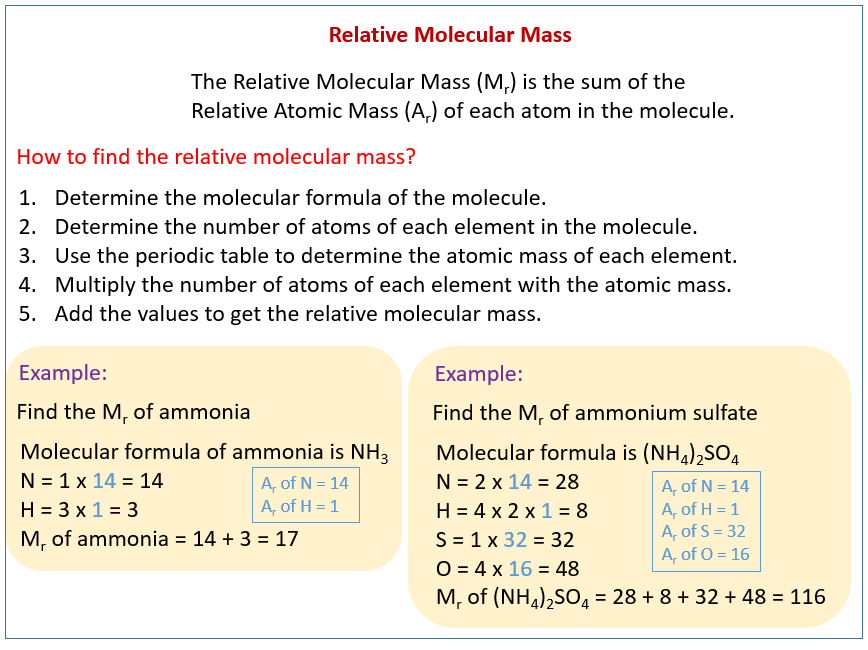
What is relative formula mass and relative molecular mass?
The relative formula mass of a substance is the sum of the relative
atomic masses of the elements present in a formula unit. The symbol for relative formula mass is Mr.
If the substance is made of simple molecules, this mass may also be called the relative molecular mass.
How to calculate the relative formula mass?
- Write the Chemical Formula:
Identify the chemical formula of the molecule (e.g., H2O, CO2). - Find the Atomic Masses:
Use the periodic table to find the atomic mass of each element in the molecule.
Atomic mass is usually listed below the element’s symbol (e.g., H = 1, O = 16.00). - Multiply by the Number of Atoms:
Multiply the atomic mass of each element by the number of atoms of that element in the molecule. - Add the Results:
Sum the masses of all the atoms to get the relative molecular mass.
Example:
What is the relative mass formula of hydrogen gas? (Relative atomic mass: H = 1)
Solution:
The formula for hydrogen gas is H2. Each molecule contains 2 hydrogen atoms.
The relative mass formula of hydrogen gas is
Mr(H2) = 2 × Ar(H) = 2 × 1 = 2
Example:
What is the relative mass formula of water? (Relative atomic masses: H = 1, O = 16)
Solution:
The formula for water is H2O. Each molecule contains 2 hydrogen atoms and 1 oxygen atom.
The relative mass formula of water is
Mr(H2O) = 2 × Ar(H) + Ar(O) = 2 × 1 + 16 = 18
Example:
What is the relative mass formula of sodium chloride? (Relative atomic masses: Na = 23, Cl = 35.5)
Solution:
Sodium Chloride is an ionic solid with the formula Na+Cl-.
The relative mass formula of sodium chloride is
Mr(NaCl) = Ar(Na) + Ar(Cl) = 23 + 35.5 = 58.5
How to find the Percent Mass of Elements in a compound?
How to use Mr to calculate the percent mass of an element
in a compound?
Example:
What percentage of the mass of ammonium nitrate is nitrogen? (The formula for ammonium nitrate is NH4NO3, Relative atomic masses: H = 1, O = 16, N = 14)
Solution:
Mr(NH4NO3) = (2 × 14) + (4 × 1) + (3 × 16) = 28 + 4 + 48 = 80
Mass of nitrogen in the formula = 28
Mass of nitrogen as a fraction of the total = <imgloading=“lazy” src="/image-files/chem37.gif" width=“24” height=“41” align=“absmiddle”>
Mass of nitrogen as percentage of total mass ![]()
Example:
What percentage of the mass of O in NaNO3? (Relative atomic masses: Na = 23, O = 16, N = 14)
Solution:
Mr(NaNO3) = 23 + 14 + (3 × 16) = 23 + 14 + 48 = 85
Mass of O in the formula = 48
Mass of O as a fraction of the total = ![]()
Mass of O as percentage of total mass ![]()
How to find percent by mass and percent composition?
Example:
Find the percent composition by mass of potassium dichromate (K2Cr2O7)
Step 1: Find the molar mass of the compound.
Step 2: Divide the total mass of each element by the molar mass and multiply by 100 to find the % mass.
How find the percent of an element in a compound?
Finding the percent by mass means finding the mass of the elements in the compound and adding the masses
for the total mass.
Example:
You have a 7364 milligrams sample of SO2. How many grams of sulfur are in the sample?
How to calculate the Mass Percent of an Element in a Compound?
Mass Percent Composition of an Element in a Compound
To calculate the mass percent composition (or simply, the mass percent) of an element in a compound,
we divide the mass of the element in 1 mol of the compound with the mass of 1 mol of the compound and
multiply by 100%.
Examples:
- What is the mass percent of carbon in carbon dioxide?
- What is the mass percent of oxygen in carbon dioxide?
How to find the Percent Mass of Water in a compound?
How to use Mr to calculate the percent mass of water
in a compound?
Example:
What percentage of the mass of magnesium sulfate is water? (Given that the formula for magnesium
sulfate is MgSO4•7H2O, Relative atomic masses: H = 1, O = 16, S = 32, Mg = 24)
Solution:
Mr(MgSO4•7H2O) = 24 + 32 + (4 × 16) + (7 × 18) = 246
Mr(H2O) = 2 × Ar(H) + Ar(O) = 2 × 1 + 16 = 18
Mass of water in the formula = 18 × 7 = 126
Mass of water as a fraction of the total = ![]()
Mass of hydrogen as percentage of total mass ![]()
How to calculate the Water of Crystallization?
This video outlines how to determine the percent, by mass, of water trapped in a hydrate’s crystal lattice.
Example:
What is the percent of water, by mass, in the hydrate CuSO42H2O
How to calculate the percentage of water in the formula of a hydrated compound?
Example:
Determine the % water in the following hydrates:
a) CuSO45H2O
b) CaCl22H2O
c) KAl(SO4)212H2O
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.