Molar Volume & Avogadro's Law
Related Pages
Stoichiometry Lessons
Writing Ionic Equations
Writing A Balanced Chemical Equation
Chemistry Lessons
In these lessons, we will learn the Molar Volume, Avogadro’s Law, how to calculate gas volumes given moles and grams, how to calculate moles given gas volumes and how to calculate gas volumes given the chemical equation.
Molar Volume
The molar volume is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure.
There are two standards, commonly used in schools:
- STP (standard temperature and pressure) which is 0° C and 1 atmosphere.
- RTP (room temperature and pressure) which is 25° C and 1 atmosphere.
Avogadro’s Law states that:
1 mole of every gas occupies the same volume, at the same temperature and pressure.
At STP (standard temperature and pressure), this volume is 22.4 liters.
At RTP (room temperature and pressure), this volume is 24 dm3 (liters)
We can also say:
The molar volume of a gas is 22.4 liters at STP (standard temperature and pressure).
The molar volume of gas is 24 dm3 at RTP (room temperature and pressure).
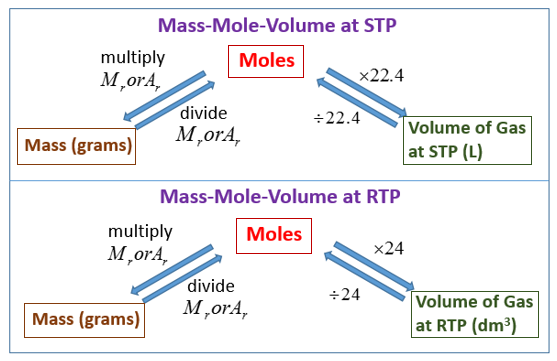
The following diagrams show how to convert between Mass, Moles and Gas Volumes. Scroll down the page for more examples and solutions.
-
Convert from Mass to Moles:
To convert from mass (in grams) to moles, you divide the mass by the molar mass of the substance.
The molar mass is the mass of one mole of a substance and is usually expressed in grams per mole (g/mol).
\(moles = \frac{mass(g)}{molar mass (g/mol)}\) -
Convert from Moles to Mass:
To convert from moles to mass (in grams), you multiply the moles by the molar mass of the substance.
mass(g) = moles × molar mass (g/mol) -
Convert Moles to Gas Volume:
To convert from moles of a gas to volume, you need to know the temperature and pressure of the gas.
At Standard Temperature and Pressure (STP)
The conversion formula at STP is:
Volume(L)= moles × 22.4L/mol
At Room Temperature and Pressure (RTP):
The conversion formula at RTP is:
Volume(L) = moles × 24.0L/mol
How to find the molar volume of a gas using the ideal gas law?
The most common molar volume is the molar volume of an ideal gas at standard temperature
and pressure (273 K and 1.00 atm).
The molar volume is the volume occupied by 1 mol of a gas at standard temperature and
pressure (STP). It can be calculated using PV = nRT.
Gas volumes from moles and grams
Example:
Calculate the volume of carbon dioxide gas, CO2, occupied by (a) 5 moles and
(b) 0.5 moles of the gas occupied at STP.
Solution:
a) Volume of CO2
= number of moles of CO2 × 22.4 L
= 5 × 22.4
= 112 L
b) Volume of CO2
= number of moles of CO2 × 22.4 L
= 0.5 × 22.4
= 11.2 L
How to convert from grams to moles to liters?
The following video shows an example of grams to moles to liters conversion.
It shows how to convert grams of a substance to liters at STP.
Example:
What is the volume of 5.643g of COs at STP?
Moles from Gas Volume
Example:
Calculate the number of moles of ammonia gas, NH3, in a volume of 80 L of the gas measured at STP.
Solution:
Volume of gas = number of moles × 22.414 L/mol
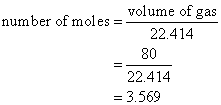
How to convert from liters to moles?
The following video shows an example of liters to moles conversion. It shows how to convert litres of a
gas at STP into moles
Example:
How many moles are there in 60.2L of COs at STP?
Gas volumes from equations
From the equation for a reaction, we can tell how many moles of a gas take part. Using Avogadro’s Law, we can also work out its volume.
Example:
What volume of hydrogen will react with 22.4 liters of oxygen to form water? (All volumes are measured at STP)
Solution:
Step 1: Write a balanced equation for the reaction.
2H2 (g) + O2 (g) → 2H2O (l)
Step 2: Calculate the volume.
From the equation, 2 volumes of hydrogen react with 1 of oxygen or
2 × 22.4 liters of hydrogen react with 22.4 liters of oxygen.
The volume of hydrogen that will react is 44.8 liters.
Example:
When sulfur burns in air it forms sulfur dioxide. What volume of this gas is produced when 1 g of
sulfur burns? (Ar : S = 32) (All volumes are measured at STP)
Solution:
Step 1: Write a balanced equation for the reaction.
S (s) + O2 (g) → SO2 (g)
Step 2: Get the number of moles from the grams.
32 g of sulfur atoms = 1 mole of sulfur atoms
So, 1 g = 1 ÷ 32 mole or 0.03125 moles of sulfur atoms
1 mole of sulfur atoms gives 1 mole of sulfur dioxide molecules
So, 0.03125 moles of sulfur atoms gives 0.03125 moles of sulfur dioxide.
Step 3: Get the volume.
1 mole of sulfur dioxide molecules has a volume of 22.4 at STP
So, 0,03125 moles has a volume of 0.03125 × 22.4 = 0.7 liters at STP
So, 0.7 liters of sulfur dioxide are produced.
How to solve equation stoichiometry questions with gases?
Examples and practice problems of solving equation stoichiometry questions with
gases. We calculate moles with 22.4 L at STP, and use molar mass (molecular weight)
and mole ratios to figure out how many products or reactants we have.
Example:
How many grams of H2O will be produced by 58.2L of CH4 at STP? Assume an excess of O2.
Examples and practice problems of solving equation stoichiometry questions with gases.
We calculate moles with the Ideal Gas Law, because the conditions are not at STP, and use molar mass (molecular weight) and mole ratios to figure out how many products or reactants we have.
Example:
If 85.0 g of NaN3 decomposes at 75°C and 2.30 atm, what volume of N2 will be made?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.