Stoichiometry (Calculations from Chemical Equations)
Related Pages
Stoichiometry Lessons
Molar Volume
More Lessons for IGCSE Chemistry
More Lessons for Chemistry
More Science Lessons (KS3/Checkpoint 1)
More Science Lessons (KS3/Checkpoint 2)
In these lessons, we will look into some examples of stoichiometry problems.
- What a chemical equation tells you?
- Does the total mass change during a chemical reaction?
- How to calculate masses from equations?
Stoichiometry is the calculation of quantitative relationships of the reactants and products in chemical reactions. Given enough information, we can use stoichiometry to calculate the moles and masses within a chemical equation.
Stoichiometry Road Map
The following Stoichiometry Road Map gives a summary of how to use stoichiometry to calculate moles, masses, volumes and particles in a chemical reaction. Scroll down the page for more examples and solutions.
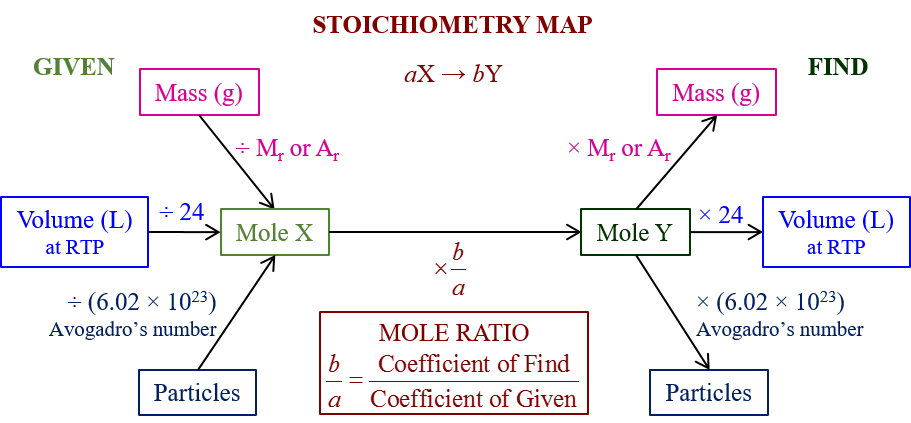
How to use the Stoichiometry Road Map?
A stoichiometry roadmap is a visual guide or a step-by-step process to solve stoichiometry problems in chemistry. It helps you navigate the conversions between different units of reactants and products in a chemical reaction. While the exact format might vary slightly depending on the source, the core principles remain the same.
1. Write the Balanced Chemical Equation:
This is the absolute first step. Without a balanced equation, the mole ratios will be incorrect, and subsequent calculations will be flawed.
Ensure that the number of atoms of each element is the same on both the reactant and product sides of the equation.
2. Identify the “Given” and the “Unknown”:
Determine what information the problem provides (e.g., mass, volume, concentration, number of particles of a reactant).
Identify what the problem asks you to calculate (e.g., mass, volume, concentration, number of particles of a product or another reactant).
3. Convert the “Given” to Moles:
The central unit in stoichiometry is the mole. You need to convert the given quantity into moles using the appropriate conversion factor:
- Mass to Moles: Use the molar mass of the substance (grams/mole) obtained from the periodic table.
- Volume of a Gas at STP: Use the molar volume of a gas at Standard Temperature and Pressure (STP), which is 22.4 L/mol.
- Number of Particles (atoms, molecules, ions): Use Avogadro’s number (6.022 x 1023 particles/mol).
4. Use the Mole Ratio from the Balanced Equation:
The coefficients in the balanced chemical equation represent the mole ratio between the reactants and products.
Set up a ratio to convert the moles of the “given” substance to the moles of the “unknown” substance.
5. Convert the Moles of the “Unknown” to the Desired Unit:
Once you have the moles of the “unknown” substance, convert it to the unit requested in the problem using the appropriate conversion factor (the reverse of step 3):
- Moles to Mass: Multiply by the molar mass.
- Moles to Volume of a Gas at STP: Multiply by 22.4 L/mol.
- Moles to Number of Particles: Multiply by Avogadro’s number.
Optional Steps (Depending on the Problem):
1. Identify the Limiting Reactant:
If the amounts of two or more reactants are given, you need to determine the limiting reactant (the one that gets used up first and limits the amount of product formed). This involves calculating the moles of each reactant and comparing their mole ratios to the balanced equation.
Calculate Percent Yield:
If the actual yield of a product is given, you can calculate the percent yield.
What a chemical equation tells you?
The equation C (s) + O2 (g) → CO2 (g) tells you that:
- 1 carbon atom reacts with 1 molecule of oxygen to give 1 molecule of carbon dioxide
- If there was 1 mole of carbon atoms then 1 mole of carbon atom reacts with 1 mole of oxygen to give 1 mole of carbon dioxide
- The moles can be changed to grams using the relative atomic mass (Ar) and the relative molecular mass (Mr)
The Ar values are: C = 12, O = 16.
The Mr values are: O2 = 32, CO2 = (12 + 32) = 44.
We can write: 12g of carbon reacts with 32g of oxygen to give 44g of carbon dioxide.
This also means that: 6g of carbon reacts with 16g of oxygen to give 22g of carbon dioxide and so on.
The masses of each substance taking part in the reaction are always in the same ratio.
In general, a chemical equation tells you:
- how many moles of each substance were involved
- how many grams of each substance were involved.
How to calculate a stoichiometry problem?
Example:
A solution containing acetic acid is mixed with calcium carbonate. How much acetic acid is consumed in a
reaction which produces 0.400 mol CO2?
Does the total mass change during a chemical equation?
Take note of what happens to the total mass, during the above reaction:
mass of carbon and oxygen at the start: 12 g + 32 g = 44 g
mass of carbon dioxide at the end: 44 g
The total mass has not changed, during the reaction. This is because no atoms have disappeared. They have just been rearranged.
This is one of the basic laws of chemistry:
The total mass does not change during a chemical reaction.
Calculating masses from equations
Example:
Hydrogen burns in oxygen to form water. What mass of oxygen is needed to burn 1 gram of hydrogen, and what mass of water is obtained?
Solution:
Step 1: Write the balanced equation for the reaction.
2H2 (g) + O2 (g) → 2H2O (l)
Step 2: Write down the relative atomic mass (Ar) and the relative molecular mass (Mr), for each substance in the equation.
Ar: H = 1, O = 16
Mr: H2 = 2, O2 = 32, H2O = 18
Step 3: Using Ar or Mr, change the moles in the equation to grams.

Step 4: Find the actual masses.
4 g of hydrogen reacts with 32 g of oxygen to give 36 g of water.
The given question states that we need to burn 1 g of hydrogen.
So, 1 g of hydrogen reacts with 32 ÷ 4 = 8 g of oxygen to give 36 ÷ 4 = 9 g of water.
Example of calculating mass from equation
Example:
Br2 + 2NaI → I2 + 2NaBr
Solving Stoichiometry Problems
In this video, we will look at the steps to solving stoichiometry problems.
- Start with your balanced chemical equation.
- Convert the given mass or number of particles of a substance to the number of moles.
- Calculate number of moles of the required substance based on the number of moles of the given substance, using the mole ratio.
- Convert the number of moles of the required substance to mass or number of particles.
Stoichiometry problems
Example 1:
How many moles of ammonia are produced by 2.8 mol of hydrogen?
Example 2:
Determine the number of moles of oxygen needed to react with 0.56 mol of vanadium to form vanadium (V) oxide?
Example 3:
Carbon dioxide reacts with lithium hydroxide to produce lithium carbonate and water.
What mass of lithium hydroxide would be required to react with 1.00 x 103 grams of carbon dioxide?
Example 4:
How many moles of silver chloride forms when 2.6 mol of KCl reacts with excess silver nitrate in solution?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.