Specific Heat Capacity
Related Pages
Specific Heat Capacity Experiment
Energy Transfers
Mechanical, Potential and Kinetic Energy
Elastic Potential Energy
Lessons for IGCSE Physics
A series of free GCSE/IGCSE Physics Notes and Lessons.
In these lessons, we will
- Describe what is meant by specific heat capacity.
- Calculate the amount of energy stored in or released from a system as its temperature change.
In physics, specific heat capacity is a fundamental property of a substance that describes how much heat energy is required to change its temperature.
Specific Heat Capacity
The specific heat capacity (c) of a substance is the amount of heat energy (Q) required to raise the temperature of one kilogram of the substance by one degree Celsius (or one Kelvin). It is expressed in units of Joules per kilogram per degree Celsius (J/kg·°C) or Joules per kilogram per Kelvin (J/kg·K).
The following diagram gives the formula for specific heat capacity. Scroll down the page for more examples and solutions on how to use the formula.
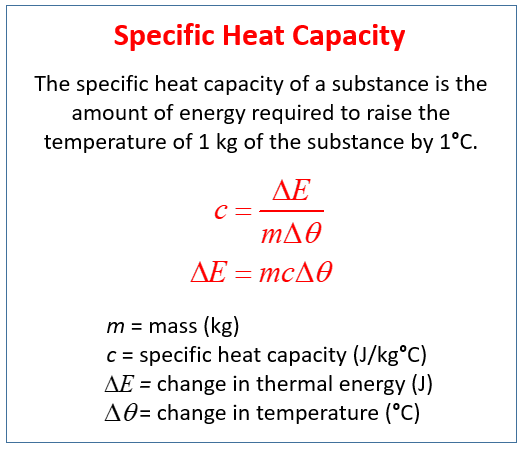
Change of Energy = m × c × change in temperature
Formula for Specific Heat Capacity
The relationship between heat energy, mass, temperature change, and specific heat capacity is given by the formula:
Q = mcΔT
Where:
Q = heat energy added or removed (in Joules, J),
m = mass of the substance (in kilograms, kg),
c = specific heat capacity (in J/kg·°C or J/kg·K),
ΔT = change in temperature (in °C or K).
Calculate Specific Heat Capacity - IGCSE Physics
Example:
A 250g copper pipe is heated from 10°C to 31°C. What is the energy needed to heat the pipe?
The specific heat capacity of copper is 390 J/kg-1°C-1.
Finding Specific Heat Capacity - IGCSE Physics
Example:
A 250g block of Aluminium is heated in a water bath to 100°C. After being placed in 300g
of 21.0°C water, the temperature of the water rises to 331°C. Find the specific heat
capacity of Aluminium.
Examples:
-
Calculate the energy required to increase the temperature of 2kg of water from 20°C to 100°C. The specific heat capacity of water is 4200 J/kg °C.
-
An iron has an aluminium plate with a mass of 1.5kg. Calculate the thermal energy stored in the plate when the temperature rises from 20°C to 200°C. The specific heat capacity of aluminium is 913 J/kg° C.
-
A hot water bottle cools down from 80°C to 20°C, releasing 756000J of thermal energy. Calculate the mass of the water in the hot water bottle. The specific heat capacity of water is 4200 J/kg°C.
Key Points About Specific Heat Capacity
- Material-Dependent:
Different materials have different specific heat capacities.
For example: Water, Aluminum, Iron - High Specific Heat Capacity:
Substances with high specific heat capacities (like water) require more energy to change their temperature.
They are good at storing thermal energy and are often used in heating or cooling systems. - Low Specific Heat Capacity:
Substances with low specific heat capacities (like metals) require less energy to change their temperature.
They heat up or cool down quickly. - Phase Changes:
Specific heat capacity applies only when the substance is in a single phase (solid, liquid, or gas). During phase changes (e.g., melting or boiling), the concept of latent heat is used instead.
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.