Solubility Curves
Related Pages
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry). These lessons look into the solubility curve.
Effects of Temperature on Solubility
Saturated solution - When a maximum amount of the solute is dissolved.
Unsaturated solution - When less than a maximum amount of the solute is dissolved.
When saturation has been reached - Rate of dissolving = Rate of crystalisation.
Solubility Curves
A solubility curve is a graphical representation that shows how the solubility of a substance (typically a solute) changes with temperature in a given solvent (usually water). It’s a powerful tool in chemistry for understanding and predicting the behavior of solutions.
The following figure shows the solubility graphs for some solids. Scroll down the page for more examples and solutions.
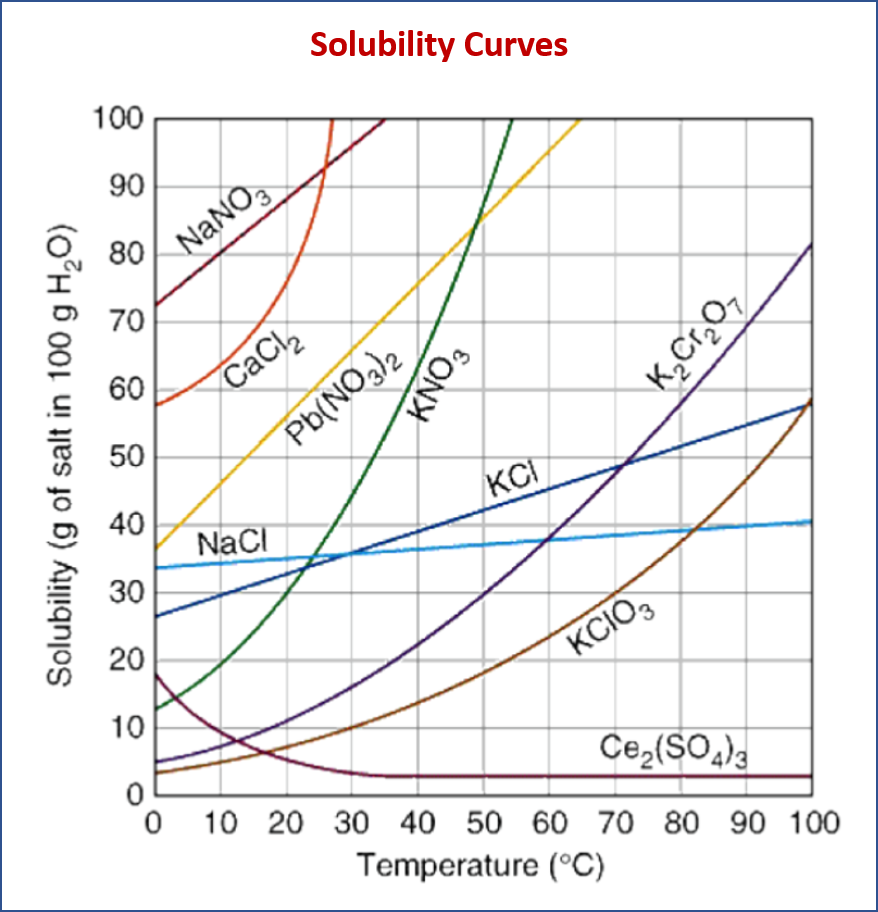
What does a Solubility Curve show?
X-axis (Horizontal): Represents temperature (usually in degrees Celsius, °:C).
Y-axis (Vertical): Represents solubility, most commonly expressed as “grams of solute per 100 grams (or 100 mL) of solvent.”
Each line on the graph represents a specific chemical substance. The curve for a particular substance indicates the maximum amount of that substance that can dissolve in the given amount of solvent at a specific temperature, forming a saturated solution.
Key Interpretations and Uses of a Solubility Curve:
- Saturated Solution: Any point on the curve indicates a saturated solution. At this temperature, the maximum amount of solute has dissolved, and if you add more, it will not dissolve and will settle at the bottom as a precipitate.
- Unsaturated Solution: Any point below the curve for a given substance indicates an unsaturated solution. This means that at that temperature, more solute could still be dissolved in the solvent to reach saturation.
Effect of Temperature on Solubility:
Most Solids: For most solid solutes, their solubility increases as the temperature increases. This is why their solubility curves typically slope upwards to the right. This is because increasing the temperature provides more kinetic energy to the solute particles, allowing them to overcome intermolecular forces and disperse more readily in the solvent.
Exceptions: There are some exceptions for solids where solubility might decrease with increasing temperature or show little change (e.g., Ce2(SO4)3 or NaCl which has a relatively flat curve).
Interpreting Solubility Curves How to read a solubility curve?
Example:
Refer to graph to answer the following questions:
- What mass of Ammonium Chloride will dissolve at 50°C in 100 g of water?
- What is less soluble in 100 g of water at 10°C sodium nitrate or sodium chloride?
- Will 100 g of potassium nitrate at 50°C in 100 g of water create a saturated solution? or unsaturated solution?
- 10 g of which substance will dissolve at 90°C in 100 g of water?
Exercise:
Refer to graph to answer the following questions:
- What mass of Sodium Chloride will dissolve at 90°C in 100 g of water?
- What mass of Potassium Nitrate will dissolve at 20°C in 100 g of water?
- What mass of Potassium Chromate will dissolve at 50°C in 200 g of water?
- What temperature is necessary for 65 g of lead nitrate to dissolve in 100 g of water?
- What temperature is necessary for 10 g of cerium sulfate to dissolve in 100 g of water?
- in 100 g of water at 60°C will 50 g of potassium chloride be saturated or unsaturated?
Questions:
The solubility curves for potassium nitrate and five solids, A, B, C, D and E, are shown for the temperature range 0°C to 100°C. The solubility is given in grams of the solid that will dissolve in 100 grams of water.
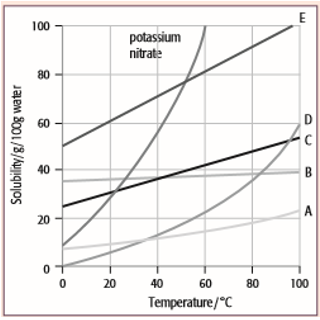
For each question, select from the graph the letter A, B, C, D or E that represents the solid described. (Each letter may be used once, more than once or not at all.)
- Which solid has the lowest solubility at 60°C?
- Which solid has the same solubility as potassium nitrate at 52°C?
- Which solid has a solubility that changes least with temperature?
- Which solid would give a deposit of 20 g if a saturated solution in 100 g of water at 60°C was cooled to 20°C?
- Which solid would be deposited in the greatest mass if a saturated solution in 100 g of water at 100°C was cooled to 80°C?
Answers
- A
- F
- B
- E
- D
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.