The Reactivity Series
Related Pages:
Reactivity of Halogens
Electronegativity
Periodic Table Trends
More Lessons for IGCSE Chemistry
More Lessons for High School Chemistry
This is a series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry). The reactivity series of metals (also known as the activity series) is a list of metals arranged in order of their reactivity, from most reactive to least reactive.
What is the Reactivity Series?
The reactivity series is a list of metals arranged in decreasing order of their reactivity. In a displacement reaction, more reactive metals displace less reactive metals from their compounds. In general, the more reactive a metal is the more vigorously it reacts with other substances and the more easily it loses electrons to form positive ions. It’s a handy tool in chemistry for predicting how metals will behave in certain reactions.
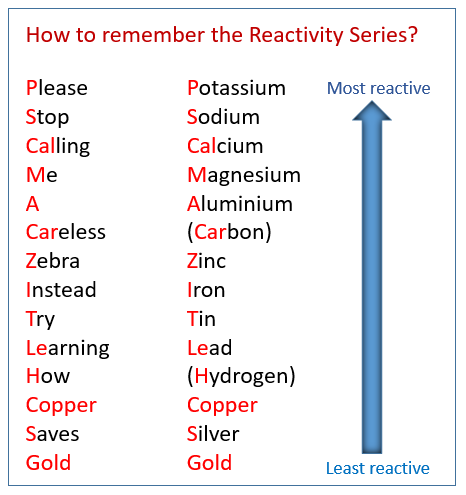
The following table shows the reactivity series of metals and how to remember them using a mnemonic. Scroll down the page for examples and solutions.
Reactivity Of Metals
How metals react with water and with dilute acids and how to use this information to order the elements by reactivity.
Explore why some metals are more reactive than other in terms of their ability to form a positive ion.
Use the reactivity series to explain the displacement of metals.
Here’s the general order of reactivity (from most to least reactive):
Potassium (K), Sodium (Na), Lithium (Li), Calcium (Ca), Magnesium (Mg), Aluminum (Al), Carbon (C), Zinc (Zn), Chromium (Cr), Iron (Fe), Tin (Sn), Lead (Pb), Hydrogen (H), Copper (Cu), Silver (Ag), Gold (Au), Platinum1 (Pt)
Key Points about the Reactivity Series:
- Metals at the top: These metals are the most reactive. They readily lose electrons to form positive ions (cations). They react vigorously with water and acids.
- Metals in the middle: These metals are moderately reactive. They react with acids, but their reaction with water is slower.
- Metals at the bottom: These metals are the least reactive. They are very stable and do not readily react with water or acids. Some of them are found in their pure form in nature (like gold and platinum).
How to Remember the Reactivity Series:
There are many mnemonics (memory aids) to help you remember the order. Here’s a common one:
“Please Stop Calling Me A Careless Zebra Cross Instead Try Learning How Copper Saves Gold, Platinum”
(Potassium, Sodium, Calcium, Magnesium, Aluminum, Carbon, Zinc, Chromium, Iron, Tin, Lead, Hydrogen, Copper, Silver, Gold, Platinum)
Uses of the Reactivity Series:
-
Predicting Displacement Reactions: A more reactive metal will displace a less reactive metal from its compound.
For example, if you put a piece of zinc (Zn) into a solution of copper sulfate (CuSO4), the zinc will displace the copper, forming zinc sulfate (ZnSO4) and solid copper (Cu):
Zn + CuSO4 → ZnSO4 + Cu
This happens because zinc is higher in the reactivity series than copper. -
Reactions with Acids: Metals above hydrogen in the series will react with acids to produce hydrogen gas.
For example, magnesium (Mg) reacts with hydrochloric acid (HCl):
Mg + 2HCl → MgCl2 + H2
Copper (Cu), being below hydrogen, does not react with dilute acids in this way. -
Reactions with Water: The most reactive metals (potassium, sodium, lithium, calcium) react vigorously with cold water.
Magnesium reacts very slowly with cold water but reacts more quickly with steam. Metals below magnesium generally do not react with water. -
Important Note about Carbon and Hydrogen:
Carbon and hydrogen are non-metals, but they are included in the reactivity series for comparison. Carbon is used in the extraction of some metals from their oxides (like iron), and hydrogen is used as a reference point for reactions with acids.
Understanding the reactivity series is essential for predicting the outcomes of many chemical reactions involving metals. It helps explain why some metals corrode easily while others remain shiny and unreactive.
Metal Displacement And The Activity Series
Metals differ in their tendency to lose electrons; more reactive metals lose electrons more easily.
A more reactive metal is able to donate electrons to the ion of a less reactive metal in a displacement reaction.
- Write equations and half-equations for reactions between a metal and the ion of a less reactive metal. Differences in metal reactivity can be represented as a metal activity series.
- Determine whether a reaction will occur between a metal and a solution containing the ions of another metal, given a metal activity series containing both metal.
Examples:
CuSO4(aq) and Zn(s)
AgNO3(aq) and Cu(s)
Predicting Displacement
Given a table of metal activity you can identify which metal will be displaced.
The more active metal is oxidised to ions.
The less active metal ion is reduced to the solid metal.
Question:
- What would occur when an iron nail is submerged in a zinc nitrate solution?
- Identify the metal being oxidised, the metal ion being reduced and write the reduction,
oxidation and overall reactions for the following pairs of metals, where a reaction occurs:
a) Chromium(s) and Sodium(aq)
b) Nickel(s) and Lead(aq)
c) Magnesium(s) and Silver(aq)
Displacement Reactions - The Reactivity Series
This activity investigates the reactions between powdered metals (magnesium, copper, iron, zinc)
and the solutions (magnesium sulphate, copper sulphate, iron sulphate, zinc sulphate). This
observational exercise allows the metals to be placed in series depending on their reactivity.
- Reaction of magnesium with magnesium sulphate, copper sulphate, iron sulphate, zinc sulphate.
- Reaction of copper with magnesium sulphate, copper sulphate, iron sulphate, zinc sulphate.
- Reaction of iron with magnesium sulphate, copper sulphate, iron sulphate, zinc sulphate.
- Reaction of zinc with magnesium sulphate, copper sulphate, iron sulphate, zinc sulphate.
Questions:
- Put the metals used (magnesium, copper, iron, zinc) in order from the least reactive to the most reactive. The more reactive metal will displace the other metal from the solution of its metal salt.
- Give the ionic equations for the reactions and explain why they are redox reactions.
- Chromium is more reactive than copper but less reactive than magnesium. Use this information to
complete the following word equations:
copper + chromium sulfate →
magnesium + chromium sulfate →
chromium + copper sulfate → - Describe how you could compare the reactivity of chromium with those of iron and zinc.
-
Show Answers
-
magnesium, zinc, iron, copper
-
Mg + Cu2+ → Mg2+ + Cu
Mg + Fe2+ → Mg2+ + Fe
Mg + Zn2+ → Mg2+ + Zn
Fe + Cu2+ → Fe2+ + Cu
Zn + Cu2+ → Zn2+ + Cu
Zn + Fe2+ → Zn2+ + Fe
The reactions are redox reactions because the oxidation states of the reactants change. -
copper + chromium sulfate → no reaction
magnesium + chromium sulfate → magnesium sulfate + chromium
chromium + copper sulfate → copper + chromium sulfate -
Add chromium to solutions of zinc ions and iron ions. Add iron and zinc metal powders to chromium ion solutions. Observe what happens.
-
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.