Naming Alkanes and Isomers
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Naming Alkanes
Alkanes are hydrocarbons with single bonds between the carbon atoms.
The general formula is CnH(2n + 2)
Alkanes and their Isomers
Isomers are compounds with the same molecular formula but different structural formula.
Example:
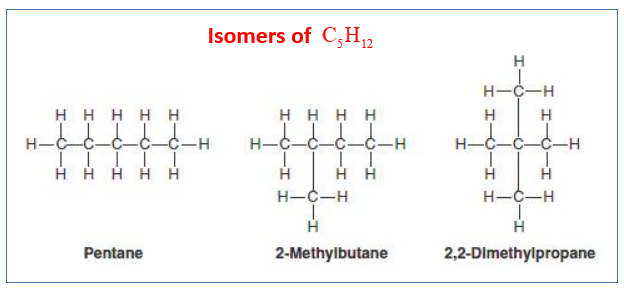
Isomers of Pentane: C5H12
n-pentane, methylbutane, dimethylpropane
Isomers of Hexane
n-hexane, 2-methypentane, 3-methypentane, 2,2-dimethybutane, 2,3-dimethybutane Questions
1. What is the key difference between the molecular and structural formula of a hydrocarbon?
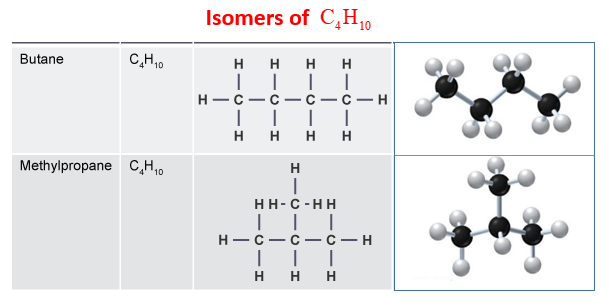
2. Name and draw the isomers of C4H10.
3. Name and draw the isomers of C5H12.
Answers



More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Naming Alkanes
Alkanes are hydrocarbons with single bonds between the carbon atoms.
The general formula is CnH(2n + 2)
Isomers are compounds with the same molecular formula but different structural formula.
Example:
Isomers of Pentane: C5H12
n-pentane, methylbutane, dimethylpropane
n-hexane, 2-methypentane, 3-methypentane, 2,2-dimethybutane, 2,3-dimethybutane Questions
1. What is the key difference between the molecular and structural formula of a hydrocarbon?
2. Name and draw the isomers of C4H10.
3. Name and draw the isomers of C5H12.
Answers
-
Show Solutions for Questions 1 and 2
1. The molecular formula shows how many of each type of atom are present in a molecule. The structural formula shows the connectivity of the atoms in the molecule - the bonds and the order in which the atoms are connected.
2.
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.