Extraction of Iron
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
These lessons look at the extraction of iron.
Related Pages
More Lessons for IGCSE Chemistry
The following diagram shows how iron is extracted from iron ore in a blast furnace. Scroll down the page for more explanations on extraction of iron.
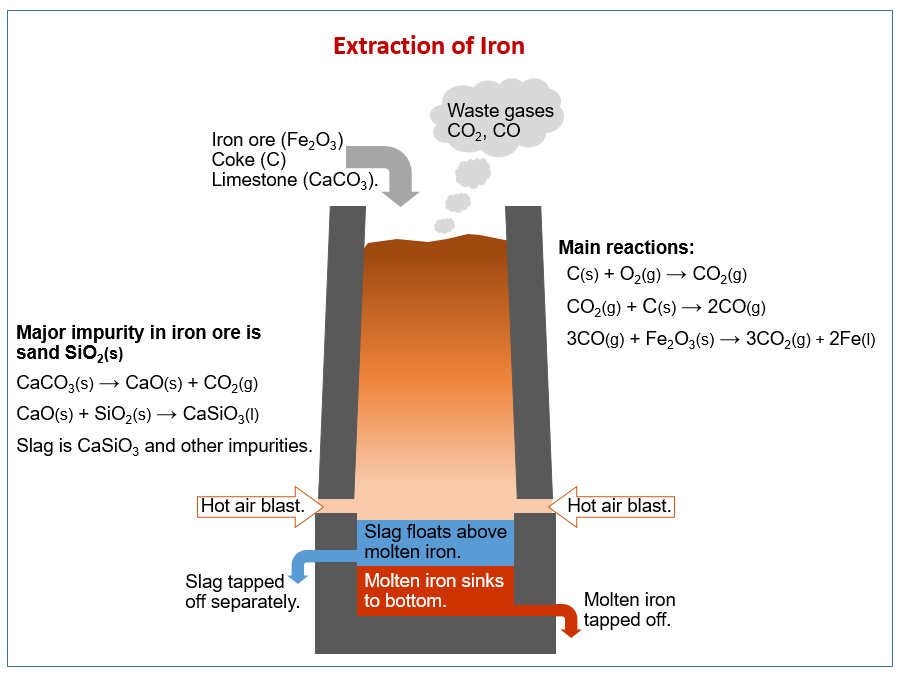
Extraction of iron
Very unreactive metals are found directly in the ground as elements, eg. gold.
Most metals are found in ores where they have reacted with other elements.
Ores are rocks containing enough metal to make it economical to extract.
We can extract the iron by reacting the iron oxide with a more reactive element.
The more reactive element takes away the oxygen.
The raw materials are Haematite (iron ore), Coke (impure C), Limestone (CaCO3)
Make a strong reducing agent
- C + O2 → CO2
- CO2 + C → 2CO
Reduce the iron 3. Fe2O3 + 3CO → 2Fe + 3CO2
Remove impurities 4. CaCO3 → CaO + CO2 5. CaO + SiO2 → CaSiO3
How to extract iron in an iron furnace?
Iron is extracted from iron ore in a huge container called a blast furnace. Iron ores such as haematite contain iron oxide. The oxygen must be removed from the iron oxide to leave the iron behind. Reactions in which oxygen is removed are called reduction reactions.
Carbon is more reactive than iron, so it can push out or displace the iron from iron oxide. Here are the equations for the reaction:
iron oxide + carbon → iron + carbon dioxide
2Fe2O3 + 3C → 4Fe + 3CO2
In this reaction, the iron oxide is reduced to iron, and the carbon is oxidised to carbon dioxide.
In the blast furnace, it is so hot that carbon monoxide can be used to reduce the iron oxide in place of carbon:
iron oxide + carbon monoxide → iron + carbon dioxide
Fe2O3 + 3CO → 2Fe + 3CO2
Iron from the blast furnace is an alloy of about 96 per cent iron with carbon and some other impurities. It is hard, but too brittle for most uses. So, most iron from the blast furnace is converted into steel by removing some of the carbon.
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.