Identify Cations
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Check with your syllabus to find which tests you will need to know for the examination.
The following table shows the flame tests for metal ions (cations):
Lithium - Crimson, Sodium - Yellow, Potassium - Lilac, Calcium - Red, Copper(II) - blue-green.
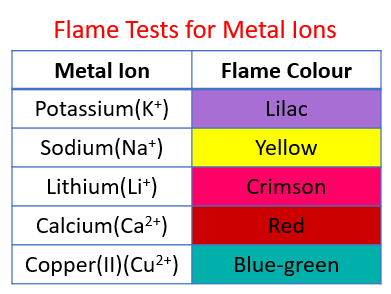
Identifying metal ions
Use flame tests to identify metal ions (cations).
Lithium - Crimson, Sodium - Yellow, Potassium - Lilac, Calcium - Red, Copper(II) - blue-green.
The following table shows the tests for aqueous cations:
Aluminium, ammonium, calcium, chromium(III), copper, iron(II), iron(III), Zinc.
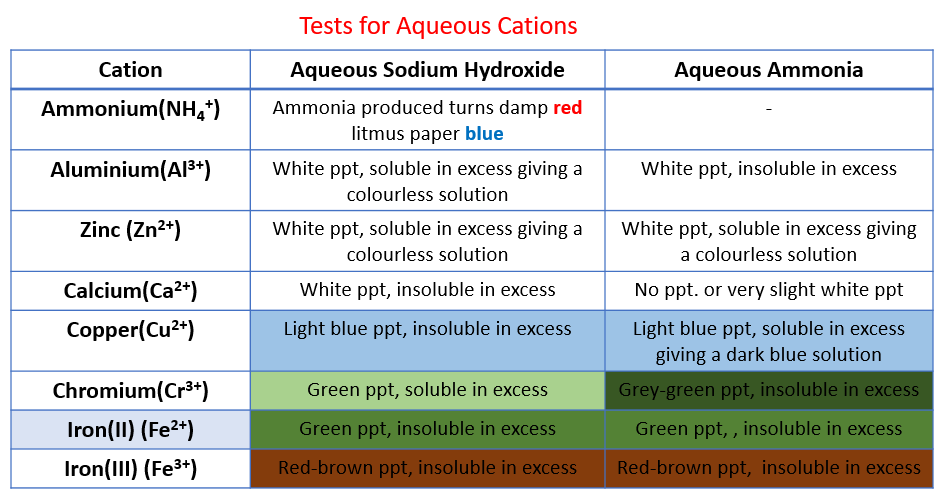
Test cations with aqueous NaOH
Sodium hydroxide is added slowly and then in excess to solutions of these salts.
The zinc (II) and Aluminium (III) form white gelatinous hydroxide precipitates that redissolve.
Copper (II) forms a light blue hydroxide that does not redisssolve.
Iron (II) and (III), respectively, form green and brown hydroxides that do not redissolve.
Test cations with aqueous Ammonia
aluminium (III) - white ppt., insoluble in excess,
calcium - no ppt. or very slight white ppt.
copper - light blue ppt., soluble in excess, giving a dark blue solution,
iron(II) - green ppt., insoluble in excess
iron(III) - red-brown ppt., insoluble in excess
zinc - white ppt., soluble in excess, giving a colourless solution
Test Chromium(III) ions with aqueous NaOH and aqueous Ammonia
grey-green ppt., insoluble in excess
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.