Homologous Series
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Homologous Series
A homologous series is a group of compounds with similar characteristics and the same general formula. Chemicals in the same homologous series will show a gradual variation in one property (eg. increasing boiling points).
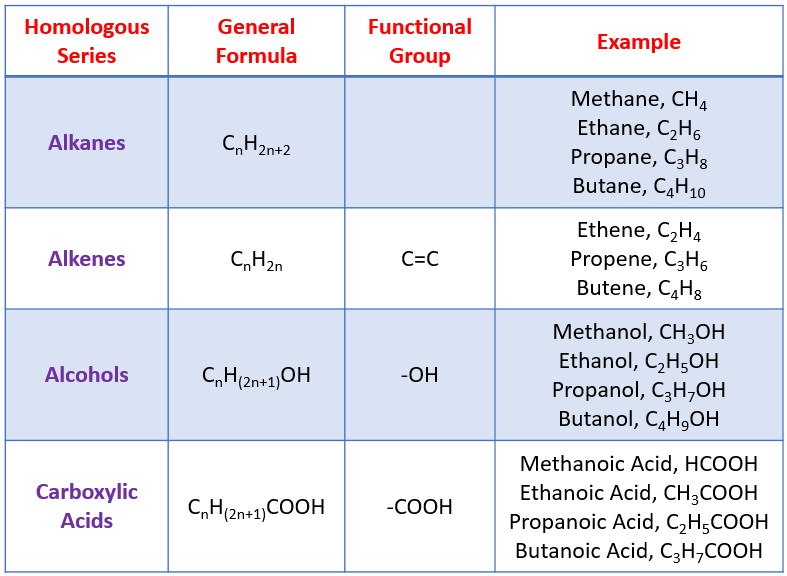
Some examples of homologous series are shown in the following table: alkanes, alkenes, alcohols, carboxylic acids.
Key Characteristics of a Homologous Series
- Same General Formula
- Same Functional Group
- Gradual Change in Physical Properties
- Similar Chemical Properties
- Successive Members Differ by a −CH2- Unit
- Similar Methods of Preparation
Alkanes are saturated hydrocarbons. Hydrocarbons are molecules that are made up of hydrogen and carbon atoms. Saturated means that all of these atoms are held together by single covalent bonds. Alkanes have the same general formula, CnH2n+2.
Alkenes contain double covalent bonds (a bond that has two shared electrons). The double bond means that alkenes have the potential to join with other molecules - this make them reactive. General formula for alkenes is CnH2n.
Alcohols are a homologous series with a functional group -OH. Some examples:
Methanol is an important raw material used in the manufacture of duels, adhesives and solvents.
Ethanol can be oxidised using agents or microbes to form ethanoic acid in vinegar (a flavouring and preservative).
General formula for alcohols is CnH2n + 1OH.
Carboxylic acids form a homologous series with the functional group -COOH. The presence of the -COOH gives carboxylic acids their properties.
Some facts about carboxylic acids:
- They dissolve in water to form weak acidic solutions.
- They react with carbonates to form carbon dioxide.
- They react with metals.
- They react with bases.
Esters are compounds with the functional group -COO-. Ethyl ethanoate is the main examples and it is formed when ethanic acid reacts with an alcohol.
Ethanoic acid + ethanol → ethyl ethanoate + water
The reaction is carried out in the presence of a catalyst (eg. concentrated sulphuric acid). They have distinctive smells and are used for perfumes and food products.
Functional Groups and Homologous Series
Homologous Series
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.