Heat and Internal Energy
A series of free Science Lessons for 7th Grade and 8th Grade, KS3 and Checkpoint Science in preparation for GCSE and IGCSE Science.
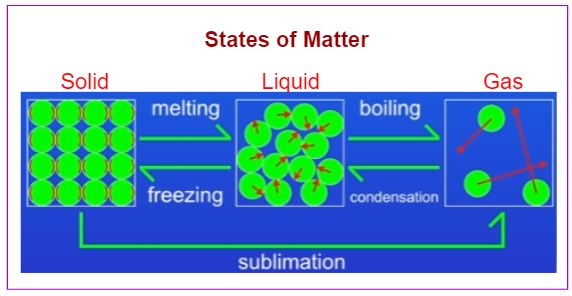
The following diagram shows the changes in the states of matter: melting, boiling, condensation, freezing, sublimation. Scroll down the page for more examples and solutions.
Heat and Internal Energy
What is meant by internal energy?
What happens to internal energy during changes of state?
Internal energy is the total kinetic energy and potential energy of all the particles that make up a system.
When we heat a solid, we increase the internal energy. At some point, the solid will turn into a liquid (melting).
If we continue to heat the liquid then the internal energy will increase. At some point the liquid will turn to a gas (boiling).
If we cool down the gas then we reduce the internal energy. At some point, the gas turns back to liquid (condensation).
If we cool the liquid down further, the internal energy is reduced even more. Eventually, the liquid turns into a solid (freezing).
Sometimes, a solid can turn directly to a gas. It is called sublimation.
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.