Group 2 - Alkaline Earth Metals
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
The following diagram shows the Alkaline Earth Metals (Group 2 Elements) and their properties. Scroll down the page for more examples and explanations.
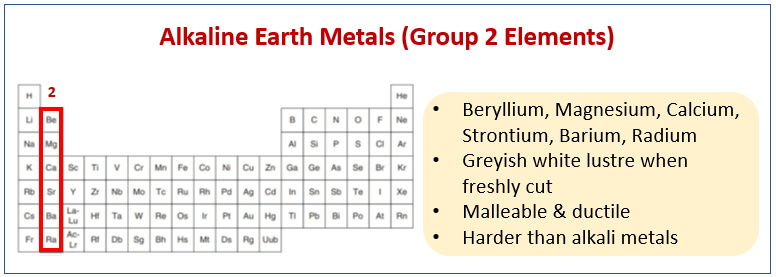
Group 2 - Alkaline Earth Metals
The alkaline earth metals found in group 2 of the periodic table. They are beryllium, magnesium, calcium, strontium, barium and radium.
Reaction of Alkaline Earth Metals with Water
Magnesium has a very slight reaction with cold water. However, the reaction soon stops because the magnesium hydroxide formed is almost insoluble in water and forms a barrier on the magnesium preventing further reaction.
Mg(s) + H2O(l) → Mg(OH)2(s) + H2(g)
However, magnesium burns in steam to produce white magnesium oxide and hydrogen gas.
Mg(s) + H2O(g) → MgO(s) + H2(g)
Calcium reacts fairly vigorously with cold water in an exothermic reaction. Bubbles of hydrogen gas are given off, and an alkaline solution of slightly soluble calcium hydroxide is formed.
Ca(s) + H2O(l) → Ca(OH)2(s) + H2(g)
The reaction gets more vigorous as you go down the group with strontium and barium.
Alkaline Earth metals are a group of chemical elements in the periodic table with very similar properties. What are those properties?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.