Group 0 and 7
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Group 0 and 7 - Noble Gases and Halogens
In this lesson, we will look at the
- Noble Gases (Group 0 Elements)
- Halogens (Group 7 Elements) Click here
Group 0 - Noble gases
The following diagram shows the Noble Gases (Group 0 Elements) and their properties. Scroll down the page for more examples and explanations.
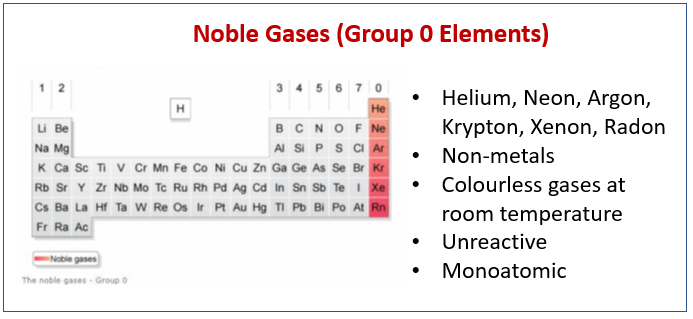
The elements in group 0 are called the noble gases or inert gases. They are found at the right-hand column of the periodic table. They consists of helium, neon, argon, krypton, xenon and radon.
The noble gases have the following common properties:
- They are non-metals.
- They are chemically unreactive gases.
- They are colourless.
- They exist as single atoms (they are monatomic).
Their boiling point and density increases as you go down the group.
Uses of noble gases
Argon provides the inert atmosphere in filament lamps because it is non-flammable. Helium can be used in airships because it has a low density.
Group 7 - Halogens
The following diagram shows the Halogens (Group 7 Elements) and their properties. Scroll down the page for more examples and explanations.

Compare the properties of the halogens
Flourine - colourless gas, most reactive
Chlorine - Pale green gas, toxic
Bromine - Orange liquid, toxic, volatile
Iodine - Dark grey solid, cystaline, sublimes forming purple vapours
- 7 electrons on outer shell
- Diatomic molecules
Describe the Halide Reactions
- Halogen react with most metals to make metal halides.
- Halogen react with hydrogen to form hydrogen halides which are water soluble and form acids.
Explain the order of reactivity and apply to displacement reactions
As you go down the group the order of reactivity decreases.
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.