Molar Mass, Grams, and Moles
Related Topics:
More Lessons for IGCSE Chemistry
More Science Lessons (KS3/Checkpoint 1)
More Science Lessons (KS3/Checkpoint 2)
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
The following diagram shows how to convert between Mass, Mole and Number of particles. Scroll down the page for more examples and explanations.
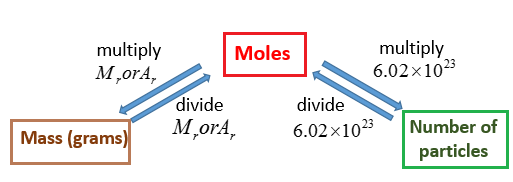
How to Calculate Molar Mass?
How to calculate the molar mass of a compound by using its chemical formula?
Molar mass is a quantity that is very similar to molecular mass, molecular weight, formula mass, and formula weight.
We will look at compounds containing polyatomic ions, and also hydrate compounds.
To calculate molar mass:
- Look at the formula to determine how many atoms of each type are in it.
- Look at the periodic table to determine the atomic mass of each of the atoms.
Examples:
Find the molar mass of:
SO2
C3H8O
Ca(NO3)2
(NH4)3PO4
MgSO4.7H2O
Convert between grams and moles
Examples:
What is the mass in grams of 4.30 moles of Aluminum?
How many moles on 12.75 grams of sodium chloride (NaCl)?
Converting Between Grams and Moles
Two approaches:
- Multiply moles by the molar mass to convert from moles to grams, and divide grams by the molar mass to convert from grams to moles.
- Conversion factor method: Write the units based on molar mass information, and set up fractions to correctly cancel the units.
Examples:
What is the mass in grams of 0.850 moles of sulfur dioxide (SO2)?
How many moles are in 32.7 grams of ethanol (C2H8O)?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.