Esters
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
The following diagram shows the reactions of alcohol and carboxylic acids to make esters. Scroll down the page for examples and explanations.
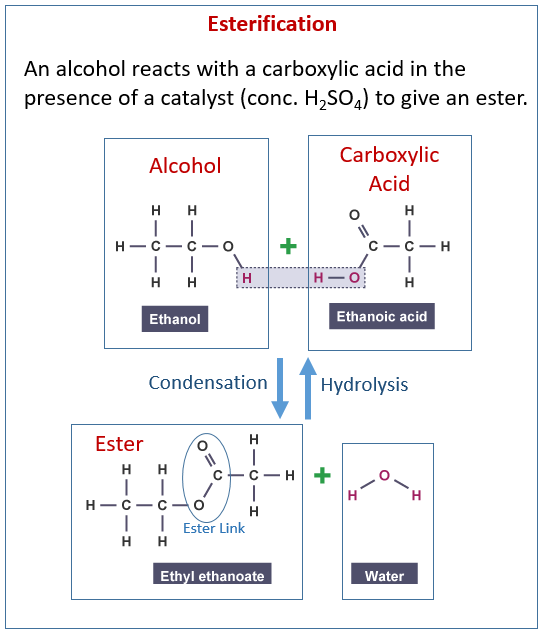
Esters
- Describe the uses of esters.
Esters are used as flavourings and fragrances. - Describe the conditions needed to produce esters.
The making of esters is also called esterification.
Esters are made from carboxylic acids and alcohols.
Ethanoic acid + ethanol ⇆ ethyl ethanoate + water
with sulphuric acid as a catalyst
CH3COOH + C2H5OH ⇆ CH3COOC2H5 + H2O - Describe the structure of the ester, ethyl ethanoate.
The ester functional group is -coo-.
Making esters from alcohols and acids
How to prepare an Ester from an alcohol and acid and separate by distillation?
In this activity, we will prepare a pure sample of ethyl ethanoate from ethanoic acid and ethanol.
- Add 25 cm3 of ethanoic acid to the flask.
- Add 25 cm3 of ethanol to the flask.
- Add 10 drops of concentrated sulphuric acid as a catalyst and gently swirl the contents in the flask.
- Warm the mixture in a warm bath for 15 minutes.
- The boiling point of ethyl ethanoate is 77°C.
- Set up the distillation apparatus.
- Collect the ethyl ethanoate which will distill at 77° C.
- Smell the product by gently wafting the odour towards your nose with your hand – do not put your nose near the top of the tube!
Drawing and naming esters
How to name and draw the structures for esters (which form from alkanols and alkanoic acids in the process of esterification)?
alkanoic acid + alkanol ⇆ ester (akyl alkanoate) + water
ethanoic (acetic) acid + methanol → methyl ethanoate (acetate)
methyl methaoate → methanoic acid + methanol
Questions
- What is the general word equation for an esterification reaction?
- What is the structural formula of ethyl ethanoate?
- Because their structures are made up of two molecules that combine together, many esters have predictable isomers.
For example: methyl ethanoate will be an isomer of ethyl methanoate since the both have the same molecular formula: C2H6O2
What would be an isomer of ethyl propanoate, for instance?
Answers
-
Show Answers
- carboxylic acid + alcohol → ester + water
- CH2COOCH2CH3
- propyl ethanoate
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.