Electrolysis of Concentrated Sodium Chloride
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
In this lesson, we will learn the electrolysis of brine or concentrated NaCl.
Electrolysis of Brine (Concentrated NaCl)
The electrolysis of concentrated sodium chloride (NaCl), also known as brine, is a very important industrial process called the chlor-alkali process. It’s used to produce three valuable chemicals: chlorine gas (Cl₂), hydrogen gas (H₂), and sodium hydroxide (NaOH).
The following diagram shows the electrolysis of concentrated sodium chloride solution. This reaction forms part of the chlor-alkali industry. Scroll down the page for examples and solutions.
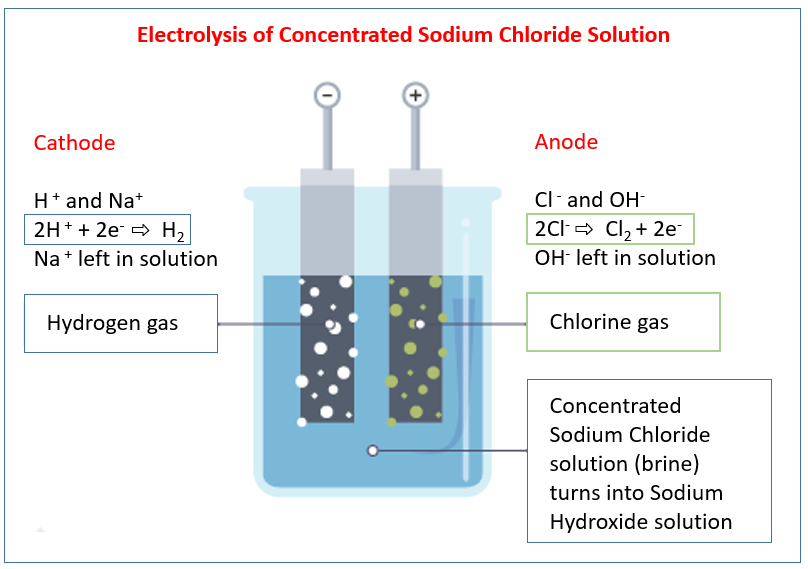
We have the following ions present in the solution:
- Na⁺ (from NaCl)
- Cl⁻ (from NaCl)
- H⁺ (from H2O)
- OH⁻ (from H2O)
Reactions at the Electrodes:
When a direct current (DC) is passed through the solution, the ions are attracted to the electrodes:
- At the Anode (Positive Electrode): Oxidation occurs
Competing Reactions: Both chloride ions (Cl⁻) and hydroxide ions (OH⁻) are attracted to the anode. However, under concentrated conditions, chloride ions are preferentially oxidized because it requires less energy.
Anode Reaction: 2Cl⁻(aq) → Cl2(g) + 2e⁻
Chlorine gas (Cl2) is produced at the anode. - At the Cathode (Negative Electrode): Reduction occurs
Competing Reactions: Both sodium ions (Na⁺) and hydrogen ions (H⁺) are attracted to the cathode. However, hydrogen ions are preferentially reduced because they are much easier to reduce than sodium ions (sodium is a very reactive metal).
Cathode Reaction: 2H⁺(aq) + 2e⁻ → H2(g)
Hydrogen gas (H2) is produced at the cathode. - Formation of Sodium Hydroxide:
As hydrogen ions are removed from the solution at the cathode, the concentration of hydroxide ions (OH⁻) around the cathode increases. The sodium ions (Na⁺) are not reduced at the cathode in this aqueous solution. The Na⁺ ions are spectator ions at the cathode. Therefore, the solution near the cathode becomes enriched with sodium ions (Na⁺) and hydroxide ions (OH⁻), resulting in the formation of sodium hydroxide (NaOH).
Electrolysis of Brine Experiment
The aim of this experiment is to investigate the products formed when a concentrated solution of sodium chloride (brine) is electrolysed.
Method
- Clamp the electrolytic cell to the stand and half-fill with concentrated sodium chloride solution.
- Fill the two small test tubes with concentrated sodium chloride solution and invert them over the electrodes as shown in the diagram.
- Connect the electrodes to the power supply using the wires and clips.
- Allow the gases to collect in the test tubes.
- Disconnect the circuit when one of the test tubes has filled with gas.
- Carry out the following tests on the gases and the solution remaining in the cell. a. Test the solution pH using Universal Indicator. b. Test the gas produced at the negative electrode with a lighted splint. c. Test the gas produced at the positive electrode with a moist piece of Universal Indicator paper. Do not breathe this gas – it is poisonous.
- Record your test results in an appropriate table.
Questions
- Comment on the reactions that have taken place at the electrodes and the solution remaining in the cell.
- Explain the change in colour of the Universal indicator.
- Name the 3 main products formed by this process and give one use of each.
- Explain why this process is economically important.
- What difference would you expect in the products formed if a very dilute solution had been used in this electrolysis?
Answers
-
Show Answers
- The solution remaining in the cell is sodium hydroxide solution. The H+ ions are discharged at the negative electrode (cathode) and the Cl- ions are discharged at the positive electrode (anode).
2H+ + 2e- → H2
2Cl- → Cl2 + 2e-
The ions H+ and Cl- are removed. Those that remain are Na+ and OH- and they form NaOH. - Sodium Hydroxide is alkaline and changes the Universal Indicator from green to purple.
- Hydrogen as a fuel, Chlorine as a disinfectant, Sodium Hydroxide to make bleach.
- Salt is cheap and abundant and can be changed by a chemical process into economically important chemicals with large-scale uses.
- When dilute NaCl solution is used, oxygen is produced at the anode from the discharge of the OH- ions.
At the anode: 4OH-(aq) → O2(g) + 2H2O(l) + 4e-
- The solution remaining in the cell is sodium hydroxide solution. The H+ ions are discharged at the negative electrode (cathode) and the Cl- ions are discharged at the positive electrode (anode).
Electrolysis of dilute sodium chloride
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.