Thermal Decomposition of Metal Compounds
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
The following table shows the thermal decomposition for metal compounds:
Nitrates, Carbonates and Hydroxides
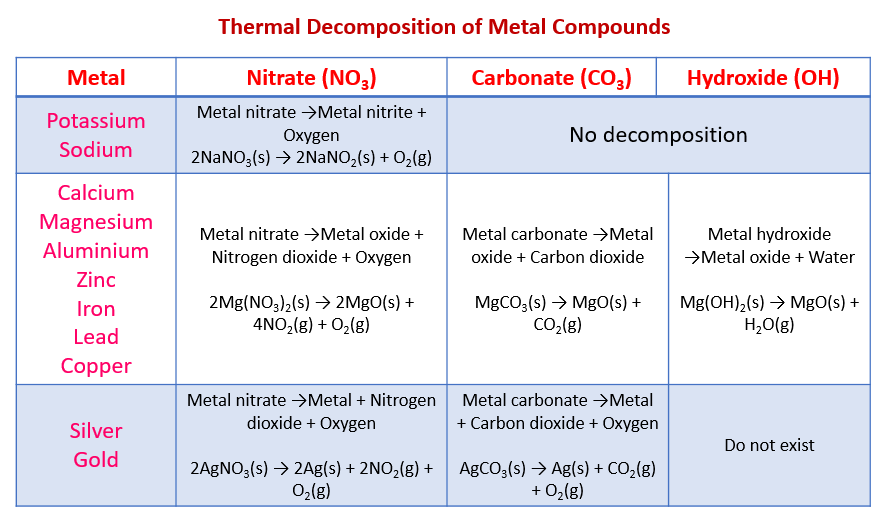
Reactivity of Metals Decomposition of Metal Nitrates
Nitrates of highly reactive metals decompose thermally to form metal nitrite and oxygen gas when heated.
Nitrates of moderately reactive metals produce brown fumes of nitrogen dioxide gas when heated, as well as the metal oxide and oxygen gas.
Nitrates of low reactive metals decompose thermally to form the metal, nitrogen dioxide and oxygen.
Thermal decomposition of metal carbonates:
Calcium, lead, copper
Decomposition of Metal Hydroxide
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.