Chemistry WCH12 May 2020 Questions & Answers
Questions And Worked Solutions For Chemistry WCH12 May 2020
Click on the following image to get the complete paper for Chemistry WCH12 May 2020. Scroll down the page for step-by-step solutions.
IAL Chemistry WCH12 May 2020 Questions - Complete Paper (pdf)
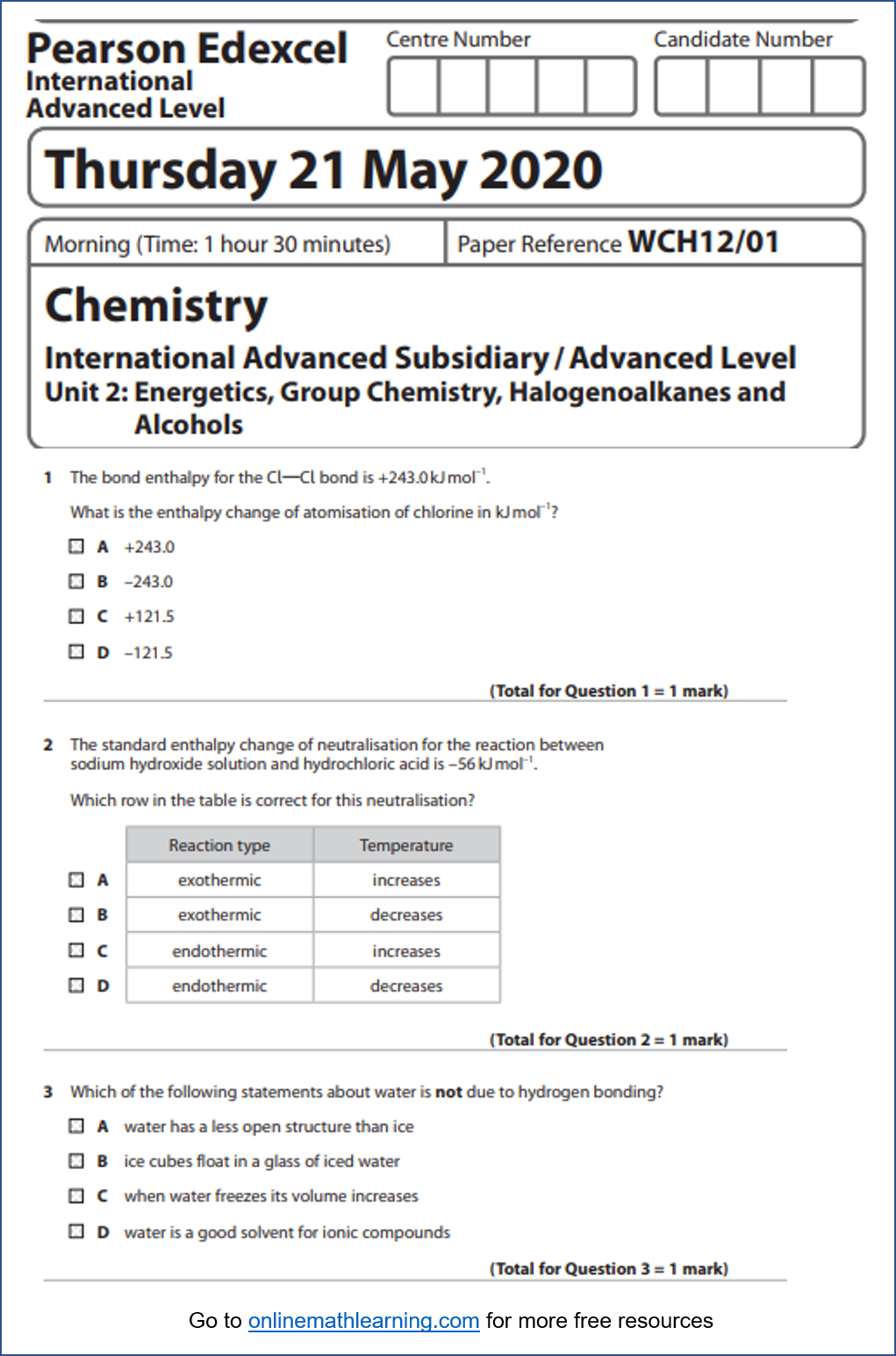
- The bond enthalpy for the Cl Cl bond is +243.0kJmol–1.
What is the enthalpy change of atomisation of chlorine in kJmol–1? - The standard enthalpy change of neutralisation for the reaction between sodium hydroxide solution and hydrochloric acid is –56kJmol–1.
- Which of the following statements about water is not due to hydrogen bonding?
- The skeletal formulae of three isomers are shown
- Hydrogen peroxide decomposes in the presence of a catalyst.
- Dichromate(VI) ions may be reduced in acidic solution.
- In an oxide of potassium, the oxidation number of oxygen is –½. What is the formula of this oxide?
- Compound Q gives off nitrogen dioxide when heated, and produces a red colour in a flame test.
- The products of the reaction of sodium fluoride with concentrated sulfuric acid can be predicted by considering the trends for the other sodium halides.
- The diagram shows a reaction profile for a reversible reaction
- A solution containing 0.100mol of hydrochloric acid is added to 8.43g of magnesium carbonate.
- (a) What is the name of compound L?
(b) Compound L can be converted into compound M.
(c) Compound L can also be converted into compound N
- Enthalpy changes of formation are often difficult to determine directly.
(a) (i) Add arrowheads and stoichiometric coefficients to the Hess’s Law diagram.
(ii) Use the data at the start of the question and your Hess’s Law diagram to calculate the standard enthalpy change of formation of propane.
Include a sign and units in your answer
(b) The values for the boiling temperatures and the standard enthalpies of combustion of a series of straight‑chain alkanes are shown in the table.
(iii) Explain, with reference to their intermolecular forces, why the boiling temperatures of alkanes increase as the number of carbon atoms increases. A detailed description of the intermolecular forces is not required. - Limewater is a solution of calcium hydroxide used in the laboratory to test for
carbon dioxide.
(a) Write the equation for the formation of the white precipitate in this test.
Include state symbols.
(b) The concentration of a saturated solution of calcium hydroxide can be determined by titration
(c) The experiment was repeated using the same hydrochloric acid with a saturated solution of magnesium hydroxide.
Explain the difference (if any) in the mean titre. - (a) Silver ions have anti-microbial properties and are used in some wound dressings. Silver nitrate can be made by warming a mixture of silver metal and concentrated nitric acid.
- Halogenoalkanes are useful reagents in organic synthesis. Some reactions of 1-bromopropane are shown.
- (a) The concentration of atmospheric carbon dioxide is at its highest level for 800000 years.
Carbon Capture and Utilisation (CCU) uses waste carbon dioxide from industrial
processes to make green fuels, methanol, plastics or pharmaceuticals.
One method uses the reaction between carbon dioxide and hydrogen to make methanol.
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.