

Chemical Reactions
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
Chemical reactions and equations, Equations for chemical reactions, Types of chemical reaction, Redox reactions, Electrolysis, Electrode reactions.
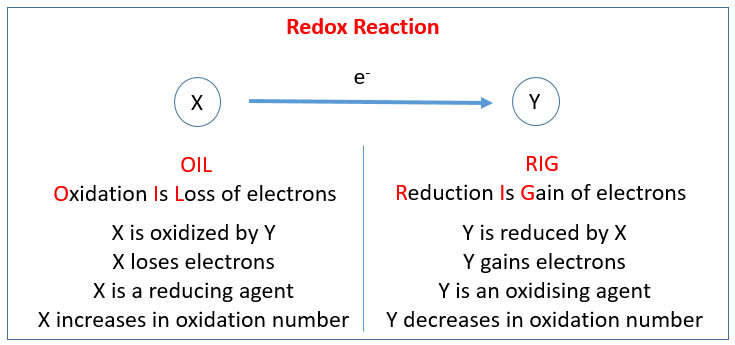
The following table shows the Redox Reaction. Scroll down the page for examples and solutions.
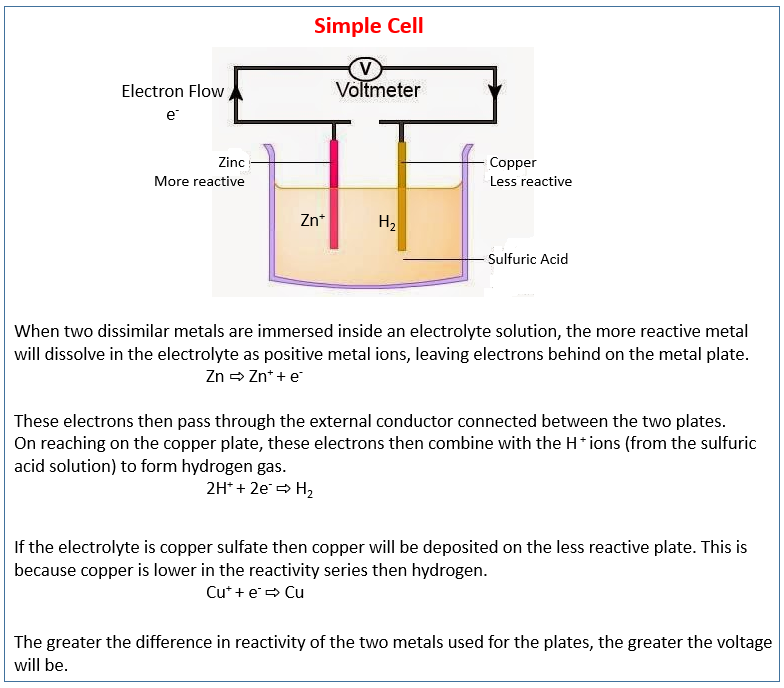
The following diagram shows the simple cell. It describes the production of electrical energy from simple cells, i.e. two electrodes in an electrolyte. Scroll down the page for examples and solutions.
Balancing chemical equations 1
Balancing chemical equations 2
Balancing chemical equations 3
Balancing chemical equations 4
Major Types of Chemical Reactions
Five major types of chemical reactions: synthesis, decomposition, combustion, single replacement (also called single displacement) and double replacement (also called double displacement).
In a synthesis reaction, a compound is made from more simple materials.
In a decomposition reaction, a compound breaks down into simpler elements or compounds.
In a combustion reaction, a compound (usually with carbon, hydrogen, and sometimes oxygen) combines with oxygen to give carbon dioxide and water.
In a single replacement (displacement) reaction, one element that is on its own displaces another element in aqueous solution, kicking it out.
In a double replacement (displacement) reaction, the positive and negative ions in two ionic compounds switch places, causing a precipitate to form. Introduction to Oxidation Reduction (Redox) Reactions
An oxidation reduction (redox) reaction happens when electrons are transferred between atoms.
A loss of electrons is called oxidation, and we say that atom has become oxidized.
A gain of electrons is called reduction, and we say that the atoms has become reduced.
The two separate parts (oxidation and reduction) of an oxidation reduction (redox) reaction are called half reactions.
Two half reactions can be put together to make the whole reaction.
Oxidation numbers are numbers that can be written above atoms to show whether they are gaining or losing electrons. Oxidizing Agents and Reducing Agents
Oxidizing agents make oxidation happen, and reducing agents make oxidation happen.
An oxidizing agent takes electrons from something, allowing it to be oxidized, and a reducing agents gives electrons to something, allowing it to be reduced.
You can remember this by noting that the thing that is reduced is the oxidizing agent, and the thing that is oxidized is the reducing agent.
We'll then look at some equations and identify the oxidizing and reducing agents. To do this, we have to write oxidation numbers (or oxidation states) for the elements in the equation, and then figure out how electrons are moving, what is being oxidized and what is being reduced.
How to Write Net Ionic Equations?
How to determine spectator ions and write a net ionic equation?
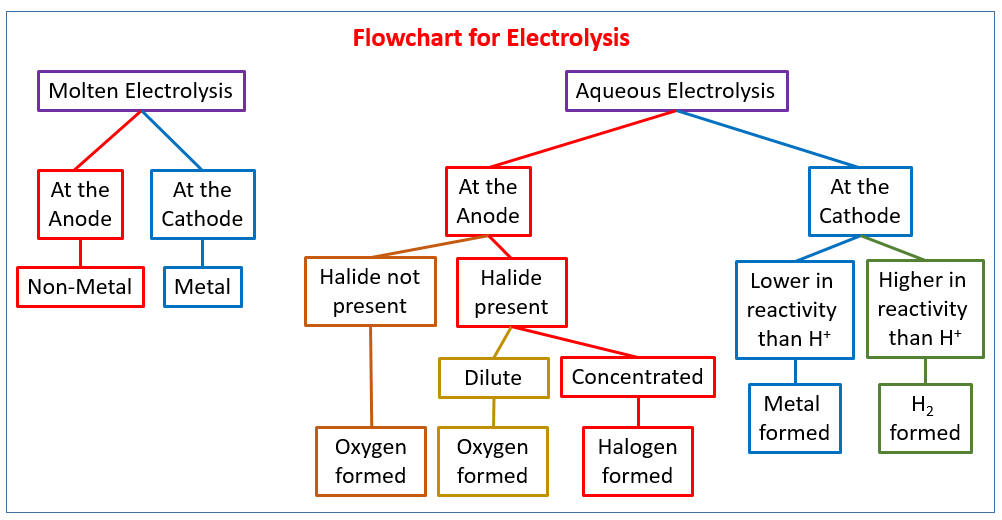
Introducing electrolysis
Electrolysis of molten lead bromide
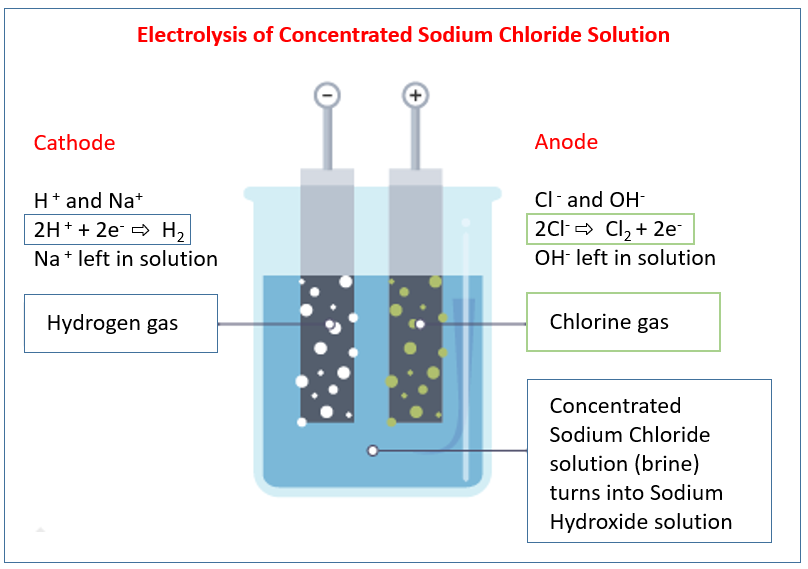
Electrolysis of concentrated sodium chloride solution
Electrolysis of dilute sodium chloride (inert electrodes)
Discussion on selective discharge during electrolysis of aqueous dilute sodium chloride, Electrolysis of aqueous copper(II) sulfate
Using copper electrodes to refine blister copper by electrolytic refining Electrolysis of aqueous copper sulfate (inert electrodes)
Discussion on selective discharge during electrolysis of aqueous copper (II) sulfate Electrolysis of aluminium oxide
Electrolysis of concentrated HCl
Electrolysis of Dilute Aqueous Sulphuric Acid
Introduction to electroplating
Example: copper coating a steel fork Purification of copper
How purify copper by the process of electrolysis Reactivity Series, Tests for Ions, Redox, Displacement



More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
Chemical reactions and equations, Equations for chemical reactions, Types of chemical reaction, Redox reactions, Electrolysis, Electrode reactions.
The following table shows the Redox Reaction. Scroll down the page for examples and solutions.
Balancing chemical equations 1
Balancing chemical equations 2
Balancing chemical equations 3
Balancing chemical equations 4
Major Types of Chemical Reactions
Five major types of chemical reactions: synthesis, decomposition, combustion, single replacement (also called single displacement) and double replacement (also called double displacement).
In a synthesis reaction, a compound is made from more simple materials.
In a decomposition reaction, a compound breaks down into simpler elements or compounds.
In a combustion reaction, a compound (usually with carbon, hydrogen, and sometimes oxygen) combines with oxygen to give carbon dioxide and water.
In a single replacement (displacement) reaction, one element that is on its own displaces another element in aqueous solution, kicking it out.
In a double replacement (displacement) reaction, the positive and negative ions in two ionic compounds switch places, causing a precipitate to form. Introduction to Oxidation Reduction (Redox) Reactions
An oxidation reduction (redox) reaction happens when electrons are transferred between atoms.
A loss of electrons is called oxidation, and we say that atom has become oxidized.
A gain of electrons is called reduction, and we say that the atoms has become reduced.
The two separate parts (oxidation and reduction) of an oxidation reduction (redox) reaction are called half reactions.
Two half reactions can be put together to make the whole reaction.
Oxidation numbers are numbers that can be written above atoms to show whether they are gaining or losing electrons. Oxidizing Agents and Reducing Agents
Oxidizing agents make oxidation happen, and reducing agents make oxidation happen.
An oxidizing agent takes electrons from something, allowing it to be oxidized, and a reducing agents gives electrons to something, allowing it to be reduced.
You can remember this by noting that the thing that is reduced is the oxidizing agent, and the thing that is oxidized is the reducing agent.
We'll then look at some equations and identify the oxidizing and reducing agents. To do this, we have to write oxidation numbers (or oxidation states) for the elements in the equation, and then figure out how electrons are moving, what is being oxidized and what is being reduced.
How to Write Net Ionic Equations?
How to determine spectator ions and write a net ionic equation?
Introducing electrolysis
Electrolysis of molten lead bromide
Electrolysis of concentrated sodium chloride solution
Electrolysis of dilute sodium chloride (inert electrodes)
Discussion on selective discharge during electrolysis of aqueous dilute sodium chloride, Electrolysis of aqueous copper(II) sulfate
Using copper electrodes to refine blister copper by electrolytic refining Electrolysis of aqueous copper sulfate (inert electrodes)
Discussion on selective discharge during electrolysis of aqueous copper (II) sulfate Electrolysis of aluminium oxide
Electrolysis of concentrated HCl
Electrolysis of Dilute Aqueous Sulphuric Acid
Introduction to electroplating
Example: copper coating a steel fork Purification of copper
How purify copper by the process of electrolysis Reactivity Series, Tests for Ions, Redox, Displacement
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.