Chemical Formula
To write the chemical formula of a compound, we must know the valency of the elements or polyatomic ions involved.
We can determine the valency from the group number in the periodic table.
In general, the normal valency of an atom is equal to the smaller of the following two numbers
- its periodic group number, or
- eight minus its group number.
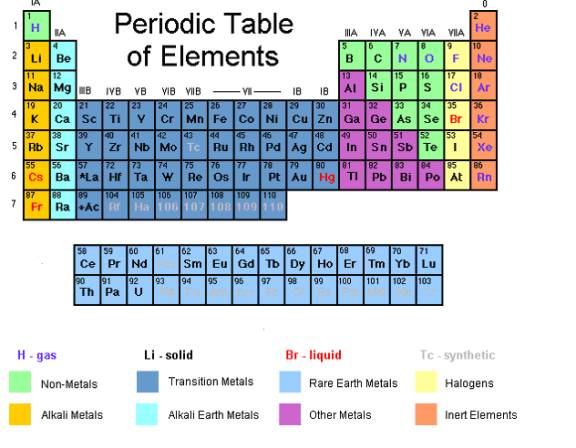
Group Number in the Periodic Table |
Example of element |
Valency |
I |
Li, Na |
1 |
II |
Be, Mg |
2 |
III |
B, Al |
3 |
IV |
C, Si |
4 |
V |
N, P |
3 |
VI |
O, S |
2 |
VII |
F, Cl |
1 |
Transition metallic elements have variable valencies. Their valencies are indicated by a Roman numeral in the bracket.
Example of transition metallic element |
Valency |
Copper (II) |
2 |
Iron (III) |
3 |
Lead (IV) |
4 |
Manganese (VI) |
6 |
There are radicals which consist of a group of atoms known as polyatomic ions. The following table shows the valencies of some common polyatomic ions.
Common polyatomic ions |
|||
Positive |
Valency |
Negative |
Valency |
Ammonium ion, NH4+ |
1 |
Nitrate ion, NO3- |
1 |
Hydroxide ion, OH- |
1 |
||
Carbonate ion, CO32- |
2 |
||
Sulphate ion, SO42- |
2 |
||
Methods to deduce chemical formulas
- Write the respective valency for each element and criss-cross.
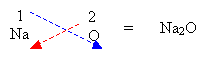
2. If the valency is equal then just write out the formula.
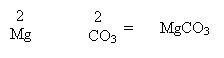
3. If a number is required for polyatomic ions then a bracket must be used.
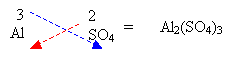
4. Reduce the ratio by a common factor if possible.
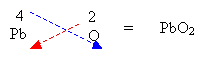
Formulas for Ionic Compounds
How to obtain the chemical formula for Aluminum Sulfide?
How to obtain the chemical formula for Iron(III) Bromide?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.