Calculating Molarity
This is a series of lectures in videos covering Chemistry topics taught in High Schools. These lessons look at how to calculate moles and molarity.
Related Pages
Mole Calculation
Molar Volume
Stoichiometry Lessons
Molecular Mass
Chemistry Lessons
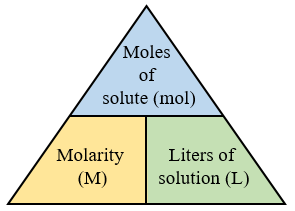
The following diagram shows how to convert between Molarity, Moles and Volume. Scroll down the page for more examples and solutions.
Molarity Practice Problems
Practice problems with molarity, calculate the moles and liters to find the molar concentration.
How to use conversion factors to convert between grams and moles, and between milliliters and liters.
Examples:
- Calculate the molarity of a solution prepared by dissolving 9.8 moles of solid NaOH in enough water to make 3.62 L of solution.
- You dissolve 152.5g of CuCl2 in water to make a solution with a final volume of 2.25L. What is its molarity?
- A solution has a volume of 375 mL and contains 42.5 g of NaCl. What is its molarity?
How to solve Molarity Problems?
Examples:
- How many moles of NaCl are in 3.5L of a 1.5M solution of NaCl?
- If you have 4.1 moles of glucose and want to have a 0.25 M solution with it, what will be the final volume of the solution?
- If a student has 35.0 g of FeCl3 and needs to make a 1.5 molar solution with it, what will the volume of the solution be?
- How many grams of NaOH do you have to dissolve to make 725 mL of a 2.5 M solution?
How to Calculate Molarity | Practice Problem #1
Example:
What is the molarity of a solution made by dissolving 1.461 g of NaCl in 250.0 mL of water? (MM of NaCl = 58.443 g/mol)
How to Calculate Molarity | Practice Problem #2
How many grams of AgNO3 are needed to prepare 250mL of 0.0125M AgNO3? (MM of AgNO3 = 169.87 g/mol)
Concentration Calculations 1 (molarity)
A tutorial on calculating the molarity or the concentration, of a solution.
Examples:
- If 1.25 moles of NaCl is dissolved in 250 mL of water, determine the molarity.
- If 12.0 grams of calcium bromide is dissolved in 500 mL of water, determine its molarity.
Concentration Calculations 2 (ppm)
A tutorial on calculating the concentration of a solution in parts per million.
Examples:
- Jane dissolves 2.5 grams of cleanser in 1250 mL of water to clean a water jug. Determine the concentration of the resulting solution in ppm.
- If 1.3 × 10-2 moles of Barium Nitrate is dissolved in 750 mL of water, what would the concentration be in ppm?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.