Atomic Number & Mass Number
What is Atomic number?
- The number of protons in the nucleus of an atom is called the atomic number.
- It is represented by the symbol Z
Example:
Helium has an atomic number of 2 since it has two protons in its nucleus.
Lithium has an atomic number of 3 since it has three protons in its nucleus.
What is Mass number
- The mass number is the total number of neutrons and protons in the nucleus of the atom.
- It is also called the nucleon number.
- It is represented by the symbol A
- The atomic number and the mass number of an element is usually written in the following way:
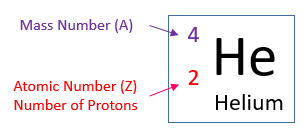
The helium atom has a mass number of 4 and atomic number of 2
Example:
What is the mass number and atomic number of the ![]() ?
?
Solution:
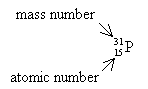
Mass number = 31, atomic number = 15
Number of neutrons = mass number – atomic number
Example:
How many neutrons are there in one atom of ![]() ?
?
Solution:
Mass number = 24
Atomic number = 12
Number of neutrons = mass number – atomic number = 24 – 12 = 12
Example:
How many neutrons are there in one atom of ![]() ?
?
Solution:
Mass number = 307
Atomic number = 82
Number of neutrons = mass number – atomic number = 207 – 82 = 125
Atomic number, mass number, and isotopes
How to use the atomic number and the mass number to represent different isotopes
The following video shows an example of calculating the number of neutrons.
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.