

Moles, Atoms, Molecules, Gases
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
The following diagram shows how to convert between Mass, Mole and Number of particles. Scroll down the page for more examples and explanations.
What is a Mole?
Converting Between Moles, Atoms, and Molecules
Examples:
How many atoms in 5.5 moles?
How many moles is 4.6 x 1024 sulfur atoms?
How to convert between moles and the number of atoms or molecules using a common sense approach, and a standard conversion factor method?
How to round the answers with scientific notation and significant figures?
More practice problems, converting between moles, atoms, and molecules
Examples: How many moles is 3.9 x 1020 Magnesium atoms?
How many molecules is 0.63 moles of molecules?
Converting Between Moles and Liters of a Gas at STP
At STP (Standard Temperature and Pressure:0° C and 1 atm), 1 mole of gas takes up 22.4 L of volume.
How to convert back and forth between moles and liters of a gas at STP?
Avogadro's Law states that 1 mole = 22.4 L at STP.
How to do calculations for the conversions?
The Ideal Gas Law must be used when a gas is not at STP, and it's important to make sure that you're dealing with a gas, and not a liquid or a solid.
Examples:
1. What is the volume in liters of 3.80 moles of CO2 gas at STP?
2. How many moles are in 58.6 L of nitrogen gas (N2) at STP?
3. What is the volume in liters of 10.3 moles of Oxygen (O2) gas at 25°C and 2 atm of pressure?
4. How many moles are in 29.4L of liquid ethanol (C2H2O) at STP?
5. What volume would 0.735 moles of O2 gas occupy at 1 atm of pressure and 0°C?
6. 13.0 liters of Chlorine gas (Cl2) at STP contains how many moles?


More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
The following diagram shows how to convert between Mass, Mole and Number of particles. Scroll down the page for more examples and explanations.
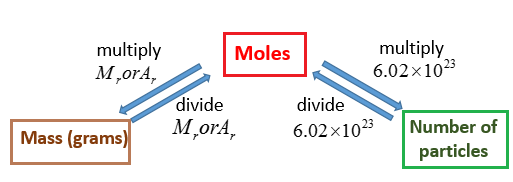
What is a Mole?
Converting Between Moles, Atoms, and Molecules
Examples:
How many atoms in 5.5 moles?
How many moles is 4.6 x 1024 sulfur atoms?
How to convert between moles and the number of atoms or molecules using a common sense approach, and a standard conversion factor method?
How to round the answers with scientific notation and significant figures?
Examples: How many moles is 3.9 x 1020 Magnesium atoms?
How many molecules is 0.63 moles of molecules?
At STP (Standard Temperature and Pressure:0° C and 1 atm), 1 mole of gas takes up 22.4 L of volume.
How to convert back and forth between moles and liters of a gas at STP?
Avogadro's Law states that 1 mole = 22.4 L at STP.
How to do calculations for the conversions?
The Ideal Gas Law must be used when a gas is not at STP, and it's important to make sure that you're dealing with a gas, and not a liquid or a solid.
Examples:
1. What is the volume in liters of 3.80 moles of CO2 gas at STP?
2. How many moles are in 58.6 L of nitrogen gas (N2) at STP?
3. What is the volume in liters of 10.3 moles of Oxygen (O2) gas at 25°C and 2 atm of pressure?
4. How many moles are in 29.4L of liquid ethanol (C2H2O) at STP?
5. What volume would 0.735 moles of O2 gas occupy at 1 atm of pressure and 0°C?
6. 13.0 liters of Chlorine gas (Cl2) at STP contains how many moles?
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.