Writing A Balanced Ionic Equation
In these lessons, we will learn how to write balanced ionic equations.
Related Pages
Writing Chemical Equations
Molar Volume, Avogadro’s Law
Chemistry Lessons
How To Write An Ionic Equation From A Word Equation?
When writing an ionic equation, state symbols of the substances must be clearly indicated. Only ionic compounds which are soluble in water (forming aqueous solution) will dissociate into ions in water. Insoluble substance cannot dissociate into ions in water.
The following diagram shows how to write the ionic equation for the reaction of aqueous sodium carbonate with aqueous barium nitrate. Scroll down the page for more examples and solutions on writing ionic equations.
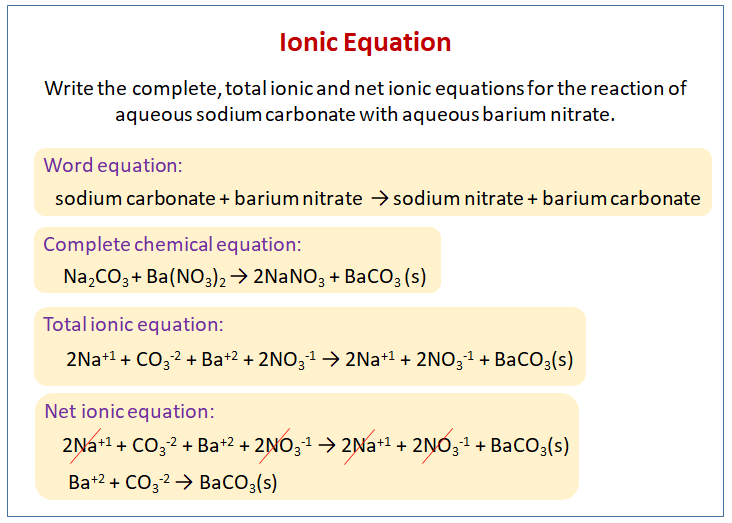
Example:
Write the ionic equation for the word equation
Sodium chloride(aq) + silver nitrate(aq) → silver chloride(s) + sodium nitrate(aq)
Solution:
Step 1: Write the equation and balance it if necessary
NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq)
Step 2: Split the ions. (Only compounds that are aqueous are
split into ions.)
Na+(aq) + Cl-(aq) + Ag+(aq) + NO3-(aq) → AgCl(s) + Na+(aq) + NO3-
Step 3: Cancel out spectator ions. (Spectator ions are ions that remain the same in their original states before and after a chemical reaction.)
Step 4: Write a balanced ionic equation
Ag+(aq) + Cl-(aq) → AgCl(s)
Example:
Write the ionic equation for the word equation
Sodium(s) + hydrochloric acid(aq) -> sodium chloride(aq) + hydrogen(g)
Solution:
Step 1: Write the equation and balance it.
2Na(s) + 2HCl(aq) -> 2NaCl(aq) + H2(g)
Step 2: Split the ions. (Only compounds that are aqueous
are split into ions.)
2Na(s) + 2H+(aq) + 2Cl-(aq) → 2Na+(aq) + 2Cl-(aq) + H2(g)
Step 3: Cancel out spectator ions. (Spectator ions are ions that remain the same in their original states before and after a chemical reaction.)
Step 4: Write a balanced ionic equation
2Na(s) + 2H+(aq) → 2Na+(aq) + H2(g)
How To Write An Ionic Equation?
Example:
Zinc + Hydrogen Chloride → Zinc Chloride + Hydrogen
How To Write Ionic And Net Ionic Equations?
Example:
Write a complete, total ionic and net ionic equations for the reaction of aqueous sodium carbonate with aqueous barium nitrate.
Molecular, Complete Ionic, and Net Ionic Equations
How To Write A Net Ionic Equation (Double Replacement)?
Basic lesson on molecular equations, complete ionic equations, and net ionic equations. All of them are technically correct, but each one is meant to show a different thing.
Example:
AgNO3 + NaBr → AgBr + NaNO3
HCl + KOH → H2O + KCl
Practice Writing Net Ionic Equations
The examples in the video are these:
Ca(NO3)2 + KF (Calcium Nitrate + Potassium Fluoride)
BaCl2 + H2SO4 (Barium Chloride + Sulfuric Acid)
KOH + HC2H3O2 (Potassium Hydroxide + Acetic Acid)
Sr(C2H3O2)2 + Li2S (Strontium Acetate + Lithium Sulfide)
Ca(OH)2 + Na3PO4 (Calcium Hydroxide + Trisodium Phosphate)
Single Replacement Reactions And Net Ionic Equations
How to write the products of a single replacement reaction and find the net ionic equation?
Examples and practice problems
Al + CuCl2 (Aluminum + Coper Chloride)
Zn + HCl (Zinc + Hydrochloric Acid)
Cl2 + NaBr (Chlorine + Sodium Bromide)
Fe + ZnCl2 (Iron + Zinc Chloride)
Na + HF (Sodium + Hydrofluoric acid)
Precipitation Reactions And Net Ionic Equations
How to balance and predict the products of precipitation reaction in addition to writing the net ionic equation?
Notes, examples, and practice problems.
Examples:
AgNO3 + CaCl2 (Silver Nitrate + Calcium Chloride)
Pb(NO3)2 + NaBr (Lead Nitrate + Sodium Bromide)
Net Ionic Equation Examples And Answers
MgCl2 + AgNO2 (Magnesium Chloride + Silver Nitrate)
H2SO4 + NaOH (Sulfuric acid + Sodium Hydroxide)
HF + KOH (Hydrofluoric acid + Potassium Hydroxide)
Na2CO3 + HCl (Sodium Carbonate + Hydrochloric acid)
Ca(NO3)2 + Na2PO4 (Calcium Nitrate + Sodium Phosphate)
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.