Elements and Compounds
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
The Periodic Table, Trends in groups, Trends across a period, Chemical bonding in elements and compounds, Chemical formulas of elements and compounds, Metals, alloys and crystals.
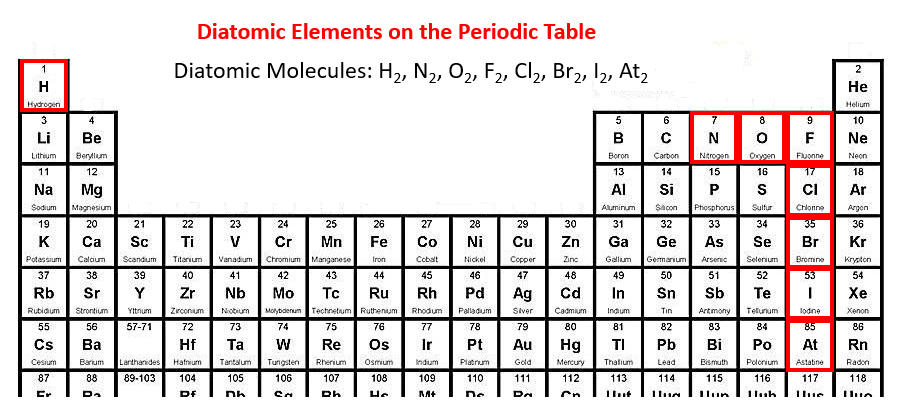
The following periodic table highlights the diatomic elements: hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, iodine and astatine.
To help you remember: the diatomic elements form a seven starting from nitrogen (element number 7).
Another way to remember: the diatomic elements names end with “gen” or “ine”.
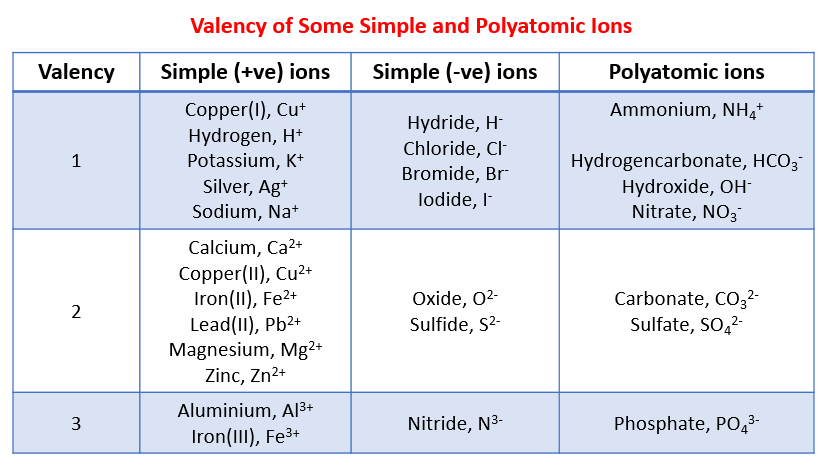
The following table shows the valency for some simple positive and negative ions and polyatomic ions. Scroll down the page for examples and solutions.
The Periodic Table
The elements in the same group of the Periodic table have the same number of outer electrons. This means that they react in the same way.
Explain why elements in group 0 do not react.
Group 0 elements are unreactive gases. They all have a full outer-energy level, which is stable.
Learn who made the periodic table, why he arranged the elements this way and about the important groups you need to know.
Properties of Metals and Non Metals
The difference between the properties of metals and non-metals, and transition metals.
Chemistry of Groups 1, 7 and 0
Properties of Group 1: The Alkali Metals,
Group 7: The Halogens,
Group 0: The Noble Gases
Trends in the Properties of Elements of Period 3
Transition elements (metals)
Explore the properties of transition elements and also their reactivity (in comparison to alkali metals).
Transition metals all have similar and very useful physical and chemical properties. Their atoms can form ions with a variable charge which also gives them a range of colours in their compounds.
Ionic bonding
Ionic bonds form when one atom transfers electron/s to another atom, so that both atoms forms oppositely charged ions. These ions are then attracted to each other by electrostatic forces which we call an ionic bond.
Covalent bonding
How covalent bonding works, how to show it with dot and cross diagrams, and the types of substances that covalent bonds can form.
Chemical bonding
How ionic, covalent and metallic bonds are formed?
How to draw dot-and-cross diagrams to represent bonding?
Properties of Ionic Compounds
Giant ionic lattices and how these determine two key properties of ionic compounds.
How ionic compounds form a giant ionic lattice and why they have high melting and boiling points?
Why they cannot conduct electricity as solids but can when they are molten or dissolved in water?
Properties of simple covalent molecules
Why small covalent molecules are almost all gases at room temperature, in terms of the intermolecular forces?
Why small covalent compounds do not conduct electricity.
Properties of Giant Covalent Molecules
What is meant by giant covalent and the structures and properties of diamond and silicon dioxide.
Formula of Ionic Compounds
How to determine the formula of an ionic compound given the charges on the ions?
Crossover method of writing formulas for ionic compounds
Structure of substances
How the properties of substances are related to the their structure and the kind of bonding they have?
Giant ionic structures, simple molecules, giant covalent structure, giant metallic structure.
Metals and Alloys
Properties of metals and of alloys and how they relate to the structures.
Examples and properties of Alloys
Stainless steel and Brass
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.