Calculate Empirical Formula
This is a series of lectures in videos covering Chemistry topics taught in High Schools.
The following diagram gives the steps to calculate the empirical formula when given the mass percentages. Scroll down the page for more examples and solutions.
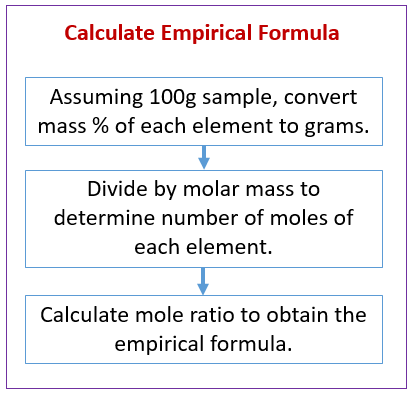
How to Calculate Empirical Formula from Mass Percentages?
Example:
A white powder used in paints, enamels and ceramics has the following percentage composition: Ba(69.6%), C(6.09%) and O(24.3%). What is the empirical formula?
Calculate Molecular Formula Given Molar Mass and Empirical Formula
How to Calculate Molecular Formula given molar mass and Empirical Formula?
Example:
Molar mass is 116.24 g/mol and empirical formula is C3H8N. What is the molecular formula?
How to Calculate Empirical Formula from Mass Data?
Example:
One of the compounds of iron and oxygen occurs naturally in the mineral magnetite. When a 2,447 g sample was analyzed it was found to have 1,771 g of Fe. Calculate the empirical formula of this compound.
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.