

Balancing chemical equations
Atoms can neither be destroyed nor created during a simple chemical reaction. Therefore, in a chemical reaction
The sum of atoms before reaction = the sum of atoms after reaction
The following figure gives some hints on how to balance chemical equations. Scroll down the page for more examples and solutions.
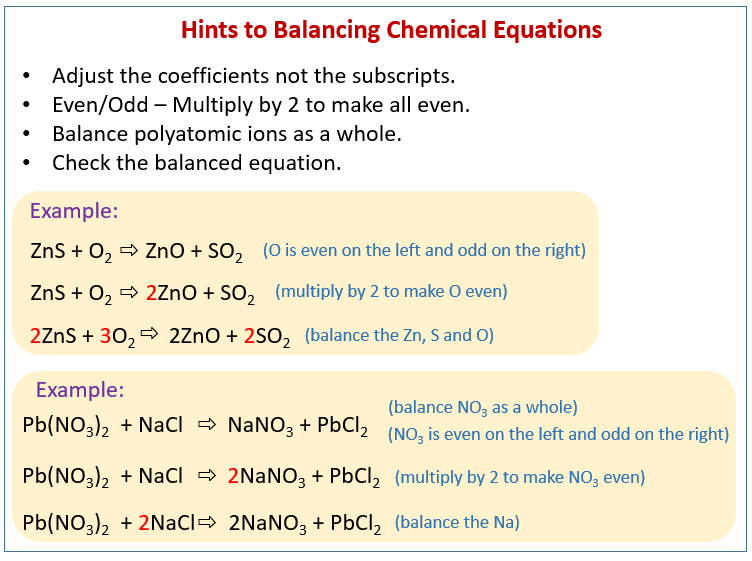
General Rules for balancing chemical equations
Balancing chemical equations may require some trial and error. There are some general rules that could be helpful, but they may not work all the time.
- Rule 1: Balancing chemical equations using the one’s and two’s technique
- Rule 2: Balancing chemical equations using the two’s and three’s technique
- Rule 3: Balancing chemical equations using the CHO technique
- Rule 4: Balancing chemical equations using the even technique
- Rule 5: Balancing chemical equations containing polyatomic ions
In this page, we will look at some examples of applying
Rule 2: Balancing chemical equations using the two’s and three’s technique.
Example:
Balance the equation
Fe + O2 → Fe2O3
Solution:
Step 1: On the left side of the equation there are 2 oxygen atoms and on the right side of the equation there are 3 oxygen atoms. To balance the oxygen atoms, multiply Fe2O3 by the coefficient of 2 and the O2 by the coefficient of 3
Fe + 3O2 → 2Fe2O3
Step 2: Balance the Fe by placing the coefficient of 4 in front of Fe
4Fe + 3O2 → 2Fe2O3
Step 3: Check that all the atoms balance and make sure that all coefficients are in the lowest-possible ratio.
Examples of using the two's and three's method to balance chemical equationsTry the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.